Four-port, no assistant port, robotic thoracoscopic surgical technique for lung lobectomies
Highlight box
Surgical highlights
• We have developed a 4-port, robotic arm only, approach to lobectomy that eliminates the need for a dedicated bedside assistant port V体育ios版. The same ports can be used to perform any lobectomy on either side.
What is conventional and what is novel/modified?
• Most robotic thoracic surgeons utilize different port placements depending on the lobe they are resecting. They usually also place another port site for a bedside assistant to manipulate the lung and to place staplers or sponges through VSports最新版本.
• Our technique eliminates the non-robotic port and standardizes the port placements for any robotic lung resection.
What is the implication, and what should change now?
• This technique simplifies the approach to robotic lung surgery by standardizing the port sites, eliminated the bedside assist role and ports, and teaching surgeons how to use the robotic staplers with maximum effectiveness.
VSports注册入口 - Introduction
Background
Lung cancer is one of the leading causes of cancer related death worldwide (1). Early-stage disease is treated by surgery alone with resection of the lung containing the tumor along with a lymph node dissection (2) VSports在线直播. Most lung cancers have been diagnosed at advance stages, when surgery does not have a role (3). Earlier detection of lung cancers through lung cancer screening and increase use of computed tomography (CT) scans in the emergency room has led to a greater number of early stage and operable lung cancers being discovered (4).
Rationale
Lung cancer surgery has historically been performed through a posterolateral thoracotomy, requiring transection of the chest wall musculature and separation of the ribs V体育2025版. This was associated with significant pain and high rates of postoperative pneumonia (5). Video-assisted thoracoscopic surgery (VATS) lobectomy was first implemented in the early 1990s and offered significant improvement in surgical outcomes and complications (6). These operations were usually performed using three or four ports, with one incision in the 6th or 7th intercostal space in the midaxillary line for the scope, second and third incisions are then made in the 3rd to the 6th intercostal space along the anterior and posterior axillary lines for additional instruments (7). Adoption of the thoracoscopic approach was slow in the early 2000s as surgeons faced challenges with low quality thoracoscopic cameras, poor lighting, and older screens.
With further advancements in technology, robotic-assisted thoracoscopic surgery (RATS), first used for pulmonary resection in 2002 (8), has become increasingly popular. Robotics allows for better visualization and improved degree of motion compared to VATS. RATS has been quickly adopted, both by traditional open surgeons and VATS surgeons (9). RATS lobectomies were initially performed using the Da Vinci S and Si systems and required the utilization of an assistant port for stapling by an experienced bedside assistant. The Xi system and robotic staplers were introduced almost 10 years ago and eliminated the need for an assistant port for stapling. Despite this, many surgeons perform surgery with only 3 robotic ports, and almost all still use a bedside assist port with a bedside assistant helping to retract the lung, suction out blood, remove specimens, or pass in sponges VSports.
While a 4-robotic-port technique has been described before (10), this technique still required a skilled bedside assistant. While having a skilled bedside assistant can be advantageous, it may be associated with higher costs relative to thoracoscopic lobectomy. When we perform thoracoscopic lobectomies, we have a surgeon and trainee at bedside, with potentially a student to hold the camera VSports app下载. When we are performing robotic surgeries, we have dual consoles with both the surgeon and trainee at a console. Having a skilled bedside assistant for these cases would be added cost for our institution (University of Michigan) and reduce our institutional support for robotic surgery.
Objective
We describe a 4-robotic-port, no assistant technique for lobectomy, using two 8-mm ports and two 12-mm ports to allow for staplers to come from multiple angles V体育官网. We use the same ports regardless of which lobe we are removing. We have the scrub technicians pass and remove instruments, specimens, and sponges, eliminating the need for an extra person at bedside.
Classification
This is a minimally invasive robotic thoracoscopic approach for curative lung resections. We present this article in accordance with the SUPER reporting checklist (available at https://vats. amegroups. com/article/view/10. 21037/vats-24-32/rc) VSports手机版.
Preoperative preparations and requirements
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments V体育安卓版. Written informed consent was not sought as this was a techniques paper that did not review patient specific results.
Robotic assisted lobectomy is performed on any patient who can tolerate an open or thoracoscopic lobectomy and has a resectable lung cancer that has not invaded into a central structure or the chest wall. Given the use of insufflation for robotic cases, it may be easier to visualize central structures using the robotic technique over the thoracoscopic one. Patients are risk stratified for surgery with adequate functional status and pulmonary function. Patients with documented lung cancer or lung nodules that are highly suspicious for being lung cancers are surgical candidates. A minimally invasive technique is preferred on patients needing a lobectomy, segmentectomy, or wedge resection. In our hands, if we suspect a pneumonectomy is needed, we perform those open as to attempt to preserve lung with a sleeve lobectomy if it can be performed.
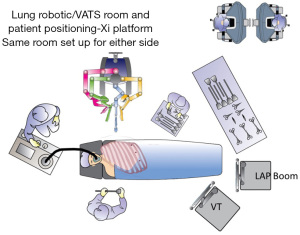
We perform our operations in a large academic medical center in the United States. We have access to multiple Da Vinci Xi systems (Intuitive Surgical, Sunnyvale, CA, USA), all with dual teaching consoles. Our lung cancer operations are performed in our Cardiovascular Center operating rooms and are staffed with a bedside surgical technician and a circulating nurse. Initial port placement is performed with a surgeon and surgical trainee, who both then scrub out to use the robotic consoles, leaving the surgical technician as the only person at the bedside until the end of the case. The anesthesia team may be composed of one anesthesiologist, or more commonly in our institution, a resident or nurse anesthetist supervised by an anesthesiologist. We feel that a surgeon requires 30–80 robotic lung resection cases to be facile with the technology, and may need lower or higher numbers, depending on their baseline comfort with thoracoscopic lobectomy. We perform all lung resections with the same ports to ensure consistency of technique for the surgeons and trainees, as well as consistency in viewing the chest and thoracic contents. We routinely perform these in 150–180 minutes, skin to skin with the trainees performing most of the console time.
"V体育2025版" Step-by-step description
VSports最新版本 - Port placement and docking
Patients are placed in a lateral decubitus position with the bed flexed to allow for maximum spread of the ribs on the operative side. The patient may be supported with a bean bag or rolled blankets, but it is ideal to have the patient’s back as far back on the bed as possible. This allows for more robot arm flexibility if needed. The patient is positioned with their back at a 90-degree angle relative to the table or leaning back 5–10 degrees. It is important to ensure the patient is not leaning forward or semi-prone, which can be a benefit when performing a thoracotomy, but can hinder a minimally invasive approach with an anterior to posterior dissection technique.
Once the patient is secure in this position. The chest is prepped to the shoulder, anteriorly to the level of the nipple, posteriorly to the spine, and inferiorly to the rib edge. After draping, the ports sites are marked out, beginning with the 8 mm camera port, usually in the 7th or 8th intercostal space along the “posterior axillary line”, based on the arms placement and pulled forward (Figure 1). Insufflation is connected to the camera port at 5 mmHg. We will call this port arm 2, reflecting the order from an anterior to posterior numbering system. The camera is placed in a manner to look along the major fissure, or one intercostal space laterally to this view. The next two ports are placed in almost a direct line with the camera angled to the patient’s back. The ports are placed 6 cm apart from each other and placement in the same intercostal space is not necessary. The most posterior port is a 12 mm port and is targeted at least 12 cm from the camera port and placed where the collapsed lung edge touches the diaphragm. The lower the port in the chest, the better for robotic stapling angles. The second 8 mm port is placed in between the posterior 12 mm port and the camera port, 1–2 intercostal spaces away from the 12 mm port. The anterior 12 mm port is then placed, as anterior as possible, in the 5th intercostal space. The skin incision may be in the inframammary fold. Once the ports are placed, the insufflation is moved to the most posterior port, the furthest away from the camera.
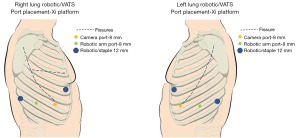
The Xi robot is docked to the camera port. It can be positioned from either side of the bed for either side of the patient (Figure 2). The robot is then targeted with the focused view on the pulmonary artery (PA), or where the PA should be, using the major and minor fissure to identify its expected location. The targeting process allows the arms to rotate and for the boom height to optimize. The arms are then docked. Prior to instrument placement, we review the depth of all non-camera ports to ensure that the “pivot” point on the ports is at the level of the ribs. Once this depth is verified, the robotic instruments are placed, with a Maryland bipolar in the most anterior 12 mm port (arm 1), a Prograsp in the other 8 mm port (arm 3), and a Tip-Up in the most posterior 12 mm port (arm 4). Our preference is to have the energy in the anterior port, which is the right hand for a right sided procedure, or the left hand, for a left sided procedure.
Bedside assist roles/placement of instruments
As we have shifted our techniques to minimize the need for an experienced bedside assist, we have focused on a few specific “rules” to minimize accidents and injuries due to less experienced trainees, surgical technologists, or nurses helping at bedside. All “exchanges” occur through the anterior 12 mm port. The bedside team places cigar rolls through this port, as well as retrieve lymph nodes through this port with a laparoscopic spoon biopsy instrument, which will fit through a 12 mm port. The surgeon can facilitate the safe placement of rolls or lymph nodes being removed by placing the Maryland arm in a safe position where the robot arms are separated on the outside and that the arm is not aimed at a critical structure, in case the assistant pushes their instrument in too far. Although the posterior 12 mm port can also be used for these situations, we use the Tip-Up through this arm for lung retraction and it is often not actively engaged (like the anterior arm). Exchanges from this port become higher risk as the surgeon must then remember to move the Tip-Up instrument from its retraction role.
Lung manipulation and lymph node dissection
The Tip-Up from the posterior 12 mm port is used for 4 major “moves”. First, the lower lobe is lifted to expose the inferior pulmonary ligament. As the ligament is mobilized, the lower lobe is grasped closer to the ligament to provide optimal tension. Station 8 and 9 lymph nodes are identified and removed during this dissection. The Prograsp is free to help make finer adjustments to tension and manipulation to the lower lobe while the Maryland is used to dissect the ligament. The 2nd major move is to push the lung anteriorly to expose the subcarinal space. The long “tip” of the Tip-Up is placed parallel to the descending azygous vein (or descending aorta) on the posterior lung, just anterior to the pleural edge. The Prograsp arm may then come in over or sometimes under the Tip-Up arm to help facilitate the mobilization of the posterior hilum and resection of the station 7 lymph nodes. The third motion is to retract the lung posteriorly, to expose the anterior hilum. The Tip-Up comes underneath the hilum and can “hook” around the upper lobe (UL) to pull the lung “back” and “down”. This motion can give exposure to the anterior and superior hilum and may be adequate for station 4R (on the right) and stations 5/6 (on the left). The last motion is performed by using the Tip-Up to pull the UL “down” to the feet to better expose the superior hilum and the proximal azygous vein on the right, and the aortic arch on the left.
Anterior to posterior dissection technique (VSports在线直播)
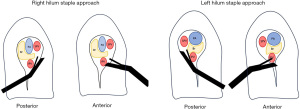
The traditional “open” lobectomy technique often begins with a fissure dissection and control of the PA with dissection then moving to the airway and pulmonary veins (PVs). Most minimally invasive thoracoscopic surgeons have learned the “anterior to posterior” technique with mobilization and division of the PV branch of the specific lobe, followed by the PA or bronchus and finishing with the final anatomic structure. The robotic approach offers more flexibility in being able to start in the fissure sometimes with better control compared to thoracoscopy. In our practice, we usually begin with the pulmonary vessels. We will outline the approach for retraction, dissection, and stapling for each specific lobe. Figure 3 demonstrates the angles for the staplers from the anterior and posterior sides. We have used a moistened umbilical tape, cut to 12 cm, to retract structures once they are dissected, and have used both the curved and non-curved staplers. We have used 45 mm staplers, but 30 mm could be used also; 60 mm staplers are too long for most patients to adequately angle the stapler safely on the hilar structures.
Right upper lobectomy (V体育官网入口)
The Tip-Up is used to retract the middle and ULs posteriorly, exposing the anterior hilum. The Maryland is used to dissect the 1 mm deep pleura lying over the superior PV. The Prograsp in the left hand then dissects the UL branches of the PV. Once this tissue plane is opened, the Prograsp is moved up and down to create room for the anvil of the robotic (or handheld stapler). The Prograsp is then removed and used to retract the right upper lobe (RUL). The Tip-Up, is moved under the Prograsp and placed around the PV as the stapler will be coming from this posterior 12 port (Figure 4A). An umbilical tape is placed at this time and retracted using the Maryland. The stapler is placed from the posterior 12 mm port and has close to a straight line to staple the PV. The thinner “anvil” side is placed behind the vein. The umbilical tape is removed prior to firing the stapler to ensure that it does not get stapled in. The stapler is removed and the Tip-Up replaced. The 12 cm umbilical tape is removed as it can get lost in a small amount of blood clot.
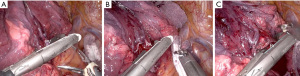
We next focus on the RUL PA branches, which could be a single trunk or 2 smaller branches. These are taken with the stapler from the posterior 12 mm port with the same retraction method as done for the vein (Figure 4B). Lastly, the bronchus is identified. If possible, the lymph node between the RUL and bronchus intermedius (BI) is freed and removed. This should allow for the Prograsp to come around the RUL bronchus. The same sequence as before of using first the Tip-Up and then the stapler from the posterior port is carried out (Figure 4C). Lastly, the minor fissure is resected using stapler firings from the anterior most 12 mm port, with the two “left” arms being used to retract the lung.
Right middle lobectomy
Similar to the retraction for the upper lobectomy, the Tip-Up is used to retract at least the right middle lobe (RML), retracting it “up” from the camera view. The major fissure between the RML and right lower lobe (RLL) is dissected free using the Maryland Bipolar or stapled partially from the anterior 12 mm port to facilitate exposure. The Prograsp is used to dissect around the RML vein branch of the superior PV. The Prograsp is then used to hold up the RML and the Tip-Up passed around the PV branch, followed by a robotic stapler from the 12 mm posterior port (Figure 5). Next, the RML bronchus is dissected out, with first the lymph node identified and removed between the RML and RLL bronchi. The same sequence is used to retract the RML and pass a stapler around it. Care must be taken with the bronchus as the RML PA branch is usually right behind this and can be small and fragile. The RML PA branch can be identified either by holding the RML vertically and coming “under” the minor fissure, or by dissecting free the minor fissure first. The remaining fissure connections (if any) is taken by a stapler from either the anterior or posterior 12 mm ports (or both).
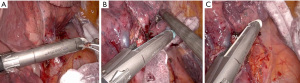
Right lower lobectomy
For this retraction, the RLL is held vertically with the Tip-Up as it was retracted for the pulmonary ligament dissection. The dissection then proceeds around the inferior PV (IPV). The Prograsp is used in the “left” hand to come around the vein anteriorly. Alternatively, a Cadiere grasper can be placed into the anterior/“right” hand port, or switch instruments and place the Maryland into the “left” hand. We prefer to use the blunter instrument to dissect around the structures. When dissecting around the IPV, care must be taken to stay “low” as to not inadvertently miss encircling the superior segment branch. Once freed, an umbilical tape is passed around the vein if needed, and the robotic stapler advanced from the anterior port. It is important to keep the wrist of the stapler “low” and aim the stapler to come almost parallel to the spine and esophagus. If the wrist is kept “high” and the stapler angled perpendicular to the esophagus, it can be harder to get the stapler fully across the vein and to clear the spine posteriorly. Once this is fired, the lower lobe continues to be retracted vertically and the posterior/inferior portion of the BI and lower and middle lobe bronchi are exposed. The lymph node between these bronchi (as outlined for the right middle lobectomy) is resected. The RLL bronchus is then encircled, again ideally using the blunter instrument from the anterior port. The PA is just behind the bronchus when retracting this way and care is taken to “err” on the side of pushing on the bronchus. The stapler for the bronchus also comes from the anterior 12 mm port. Once this is transected, the PA is exposed. It can also be mobilized and transected with a stapler from the anterior arm. If there is an incomplete fissure, this can be resected last. An alternate strategy, if there is a minimal fissure, is to mobilize the PA first and transect it with the lobe retracted inferiorly, rather than held “up”. The vein is taken afterwards as described above with the bronchus taken last.
Left upper lobectomy
The same port sites as a right upper lobectomy are used, with the same strategy of keeping them as low as possible. Here, the Maryland Bipolar comes in from the anterior 12 mm port (the left hand). For the left upper lobe (LUL), the Tip-Up is used to retract the entire lung posteriorly as described for the station 5/6 lymph node dissection. The anterior pleura is mobilized between the left superior PV and the phrenic nerve. The Prograsp is used to dissect behind the vein. Once mobilized, the robotic stapler is placed from the posterior 12 mm port, with the Prograsp used to retract the lung. After the PV is resected, the Tip-Up is again used to push the LUL posteriorly, but also to pull it down inferiorly (to the feet). This exposes the superior hilum, with the pleura dissected free. The LUL PA branches are then exposed. They are dissected free using the Prograsp and then divided with the stapler, again from the posterior 12 mm port. The “carina” between the left upper and lower lobes can be visualized once the vein is removed. The lymph node between these bronchi is removed. The LUL bronchus is then encircled. Care is taken as there is often a lingular PA branch that may still be present. When the stapler is passed, there is usually low risk if the most anterior LUL PA branches have already been resected. Once the bronchus is transected, the lingular PA branch (and any other UL PA branches) can be visualized and resected with a stapler from either 12 mm port. When retracting the LUL, care is taken to not retract too aggressively as only the fissure and small PA branches are connecting the lung to the hilum. The fissure is then resected last, usually with a stapler from the anterior port.
"V体育ios版" Left lower lobectomy
The left lower lobe (LLL) is resected with the same approach as the RLL described above. The stapler is usually passed from the anterior 12 mm port and must clear the aorta when passing it across the PV. The bronchus and PA are taken sequentially, usually with the stapler coming from the anterior port (Figure 6). The fissure, if incomplete, is also stapled from the anterior port.
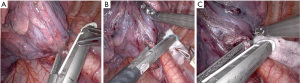
Lung removal
The robot is undocked when the lobe is freed. The camera is placed into the camera port, but the anterior port is enlarged to accommodate two fingers and a large Endo catch bag is placed through this port. The remaining 8 mm port is removed and a grasper placed to hold the lobe and to place it into the bag. The ports and equipment are all removed and the bag is slowly pulled out of the port using a circular motion. The surgeon on the posterior side holds the shoulder and hip in place and the anesthesiologist controls the double lumen tube as the bag is being removed.
Closure and pain management
After the lobe is removed, the camera port and camera are replaced. A mediastinoscopy needle is placed through the anterior most port, now enlarged, with 10 mL of local anesthetic. Each intercostal space is injected with 1 mL of anesthetic, with the needle being placed just superior to the rib to avoid injury to the intercostal nerve or vessels. The camera and port are then moved to the more posterior 8 mm port site. A chest tube suture is placed at the camera port and a 24 French chest tube placed through the original camera port site. The lung is then re-insufflated with the camera watching to ensure that there is no twisting of the remaining lobe(s). The chest tube is advanced in a posterior apical position. The deeper muscle layer for the 12 mm ports or enlarged ports are closed, followed by a deep dermal running suture, if necessary, before closing the skin with a 4-0 Monocryl suture. Skin glue is applied to the non-tube port sites and a standard dressing used for the chest tube site.
Emergency conversions
We convert to open thoracotomies approximately 5–10% of the times during a thoracoscopic (RATS or VATS) lobectomy. We have a low threshold to opening to teach our trainees safe judgement and to help our teams practice converting to open in a semi-urgent fashion, to allow for good results when urgently needed. General considerations for conversion include slow progression of the case usually due to anatomic limitations, enlarged hilar lymphadenopathy that limits visualization or mobilization of the PA, and acute bleeding from the PA, vein or bronchial artery or lymph node. When bleeding is encountered, a sponge is used and held in a robotic arm from the 3rd or 4th ports on the area of bleeding. One of the surgeons will scrub in and remove the anterior arm and port and enlarge the port site to allow a handheld sponge stick to be placed on the bleeding. The other surgeon then removes the remaining instrument and sponge off the bleeding area. The surgical technologist then removes the instruments and camera while the 2nd surgeon scrubs in and undocks the robot. The 2nd surgeon then performs a posterolateral thoracotomy, while anesthesia is maximizing resuscitation efforts, getting blood in the room, and the circulating nurse is getting help and opening the thoracotomy equipment. Once the thoracotomy is completed, headlights are placed as needed, and the bleeding can be managed through the larger incision as needed.
If there are challenges with retraction or bleeding requiring more frequent suctioning, conversion to a VATS approach may be performed, or an assistant port can be placed with one of the surgeons (trainee) functioning as the bedside assist until someone else can come assist. If the stapling is a challenge with the robotic platform, the bedside assistant or surgeon uses the 12 mm ports or an assistant port to place a handheld minimally invasive stapler. Early in our experience, we had a skilled first assist at bedside for the entire case. As we grew more comfortable with completing the operation robotically and recognizing early signs for bleeding, we began to require our First Assists to be there only for the vascular dissection, then only for the PA dissection, and now just nearby if needed at all.
Postoperative considerations and tasks
A successful lobectomy is defined by an adequate lymph node dissection, resection of the individual vascular structures, and rendering the patient cancer free. In our experience, the performance of the intercostal nerve block, used with a patient-controlled anesthesia (PCA) in the post-operative phase and early transition to oral pain medicines results in a 1–3-night stay in the hospital with discharge on oral narcotic pain medications. Immediately following extubation, patients are transferred to a recovery room with telemetry and one on one nurse to patient monitoring. Patients, once stabilized, are transferred to a telemetry floor for the remainder of their stay, where they have six to one nurse to patient care. Chest tube output is monitored every 4 hours, along with routine vitals. Chest tubes are routinely to water seal in the recovery area. Chest tubes are placed to −20 mmHg suction only if the remaining lung has collapsed, but the presence of an air leak or routine post-operative pneumothorax does not change our plan from water seal. Patients with air leaks greater than 5 days after surgery occur about 6% of the time. In these situations, the chest tube can be connected to a one-way valve or a smaller 3 chamber box system and the patient is discharged with plans for removal of the chest tube in clinic after the air leak has resolved. If the air leak is improving, we may wait 1–2 more days to try to remove the chest tube prior to discharge. Patients with chylothoraces after lung resection (<1%) are treated with thoracic duct embolization by interventional radiology. Patients with a hemothorax or empyema after lung resection (<2%) may require re-exploration and washout. Patients after surgery are started on routine beta blockers to reduce the risk of postoperative atrial fibrillation (about 10%) and routine perioperative lovenox or heparin is used to reduce the risk of veno-thromboembolism (VTE) (<2%). Patients are started on an oral diet and early ambulation the day of surgery is targeted to improve deep breathing and mucous clearance. Incentive spirometry is also encouraged multiple times a day. Once air leaks (if present) have resolved, while the chest tubes are on water seal, the chest tubes are removed, regardless of volume, if the output is serous or serosanguinous and the risk of chylothorax in the setting of higher outputs is felt to be low. The chest tubes are secured with 0-silk suture that is also used to tie down the port site where the chest tube was removed. Chest tubes are removed even in the setting of 200–300 mL per 8-hour shift without concern after a lobectomy. Patients are discharged the day of chest tube removal, or by latest the day afterwards, usually to home.
Follow up visits have historically been done in person 1–2 weeks after discharge with a repeat chest X-ray and physical examination. We remove the chest tube stitch in clinic if the family has not removed it at home. We review all our post-operative pathology results at a weekly tumor board conference where we have pathology, surgery, medical oncology, and radiation oncology present. We will determine a follow up plan which usually involves surveillance CT chest scans but may require follow up with medical oncology and/or radiation oncology. We review the patient’s pathology and long term oncologic follow up during the first post-operative visit. We try to ensure that patients are weaned off their narcotic pain medicines at this point and if they are doing well, will plan on their next follow up at 6 months for a surveillance scan and visit if indicated. If patients are still struggling with pain or functional recovery, we may see them once more in the next 4–8 weeks to re-assess their return to wellness and improved functional status. With the onset of more regular video visits and telehealth, we have now performed many of these initial visits by video, and not obtained a chest X-ray if someone is doing well. It is very common to have laxity of the oblique muscles and the upper rectus muscles on the side of the incisions due to intercostal nerves being transected with the skin incisions. We have found this to be more common after RATS compared to VATS, likely due to the lower port placement in the RATS, compared to our more variable VATS port placements.
Tips and pearls
- Consistent port placement, low in the chest with at least two 12-mm ports allows for robotic stapling of any structure in the chest from multiple angles.
- Dividing the pulmonary ligament and removing mediastinal and hilar lymph nodes early facilitates later dissection of the hilar structures.
- We do not routinely insufflate the lung after the affected bronchus is clamped as we have excellent visualization and recognition of the critical structures.
- Routine placement of the chest tubes to water seal and tolerance for chest tube removal even with higher outputs facilitate a quicker discharge and increase patient satisfaction.
VSports注册入口 - Discussion
Surgical highlights (V体育官网入口)
Our 4-robotic port technique with two 12-mm ports stationed on opposite sides of the chest allow for maximum flexibility in stapling. We have described our approach using an anterior to posterior hilar dissection technique, popularized in the VATS era to quickly and safely dissect out the hilar structures. For upper lobes bilaterally and RMLs, the staplers will usually come from the posterior 12 mm port, whereas lower lobes will usually have the staplers coming from the anterior 12 mm port. Careful dissection and use of sponges can minimize bleeding and maximize visualization. Operations can be performed consistently with short recovery times in and out of the hospital.
Strengths and limitations
The strength of our approach lies in the flexibility of being able to place staplers from any side of the patient. We also can limit the need for skilled assistants in most cases, reducing hospital costs. This could also be viewed as a limitation as we do not have skilled assistant at bedside. Other limitations with this technique include the increased postoperative abdominal wall laxity, likely due to oblique nerve innovation resection due to port sites, which is often mistaken for a hernia.
"VSports在线直播" Comparison with other surgical techniques
Overall, RATS lobectomies are associated with lower complications and conversions to thoracotomies compared to VATS lobectomies (11). The thoracoscopic uni-portal approach is a newer option with a high learning curve and increased challenges with teaching this to a bedside trainee. The camera, tools, and staplers all are placed through a single anterior port site and requires an experienced assistant to hold and manage the camera (12). The robotic single-port use for lobectomies has also been described but requires an assistant port for stapling of critical structures (13). Our 4-port robotic technique uses the most common robotic platform currently available, the Da Vinci Xi, and eliminates the need for the bedside assist and the surgeon controls the camera and all four arms from the console. This technique is particularly useful in areas where healthcare resources, including skilled workers, are scarce.
Implications and actions recommended
We would encourage any surgeon with access to an Xi platform, or the equivalent, to try this port-placement for lobectomies, but also for small lung or chest cases so that they familiarize themselves with the port sites and the viewing of the thoracic cavity with the camera placed in the anterior ports.
Conclusions
We describe our experience developing a 4-robotic port, no assistant port robotic assisted thoracoscopic lobectomy. We perform any lobectomy, segmentectomy, or wedge resection using the same 4 ports and have eliminated in our hands the routine need for a skilled bedside assist. Using the robotic platform’s staplers, we can staple any structure with the staplers coming from either the anterior or posterior ports. This may lower costs in the operating room by reducing personnel needs.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-24-32/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-24-32/prf
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-24-32/coif). R.M.R. serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from June 2024 to June 2026; he serves as a consultant and teacher for Intuitive Surgical, and as an advisory board member for Medtronic, Atricure, On Target Labs, and Genentech; he is a speaker for Bristol Myers Squib. None of these companies were involved with the writing or review of this manuscript. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was not sought as this was a techniques paper that did not review patient specific results.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- who.int. Lung cancer [Internet]. 2023 [cited 2024 Nov 13]. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Cancer.org. Can Lung Cancer Be Found Early? 2924 [cited 2024 Nov 13]. Available online: https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/detection.html
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- McKenna RJ Jr. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg 1994;107:879-81; discussion 881-2. [Crossref] [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. One hundred consecutive patients undergoing video-assisted thoracic operations. Ann Thorac Surg 1992;54:421-6. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Funai K, Kawase A, Takanashi Y, et al. Improved complete portal 4-port robotic lobectomy for lung cancer: Hamamatsu Method KAI. J Thorac Dis 2023;15:1482-5. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Sihoe AD. Uniportal video-assisted thoracic (VATS) lobectomy. Ann Cardiothorac Surg 2016;5:133-44. [VSports手机版 - Crossref] [PubMed]
- E H. Hybrid uniportal robotic-assisted thoracoscopic surgery using video-assisted thoracoscopic surgery staplers: technical aspects and results. Ann Cardiothorac Surg 2023;12:34-40. [Crossref] [PubMed]
Cite this article as: Muca A, Reddy RM. Four-port, no assistant port, robotic thoracoscopic surgical technique for lung lobectomies. Video-assist Thorac Surg 2025;10:14.


 "V体育安卓版"
"V体育安卓版"