V体育安卓版 - An optimized protocol for the generation of HBV viral antigen-specific T lymphocytes from pluripotent stem cells
- PMID: 33490980
- PMCID: PMC7806515 (VSports注册入口)
- DOI: 10.1016/j.xpro.2020.100264
An optimized protocol for the generation of HBV viral antigen-specific T lymphocytes from pluripotent stem cells
Abstract
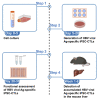
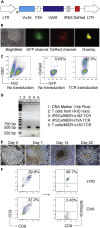
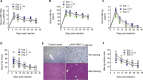
In T cell-based cancer immunotherapy, tumor antigen (Ag)-specific CD8+ cytotoxic T lymphocytes (CTLs) can specifically target tumor Ags on malignant cells. This promising approach drove us to adopt this strategy of T cell transfer (ACT)-based immunotherapy for chronic viral infections VSports手机版. Here, we describe the generation of hepatitis B virus (HBV) Ag-specific CTLs from induced pluripotent stem cells (iPSCs), i. e. , iPSC-CTLs. Ag-specific iPSC-CTLs can target HBV Ag+ cells and infiltrate into the liver to suppress HBV replication in a murine model. For complete details on the use and execution of this protocol, please refer to Haque et al. (2020). .
Keywords: Immunology; Stem Cells. V体育安卓版.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
"VSports注册入口" Figures




References
-
- Benechet A.P., Iannacone M. Determinants of hepatic effector CD8(+) T cell dynamics. J. Hepatol. 2017;66:228–233. - PubMed
-
- Gehring A.J., Xue S.A., Ho Z.Z., Teoh D., Ruedl C., Chia A., Koh S., Lim S.G., Maini M.K., Stauss H., Bertoletti A. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J. Hepatol. 2011;55:103–110. - PubMed
Publication types
- "VSports最新版本" Actions
V体育官网 - MeSH terms
- VSports注册入口 - Actions
- V体育ios版 - Actions
- Actions (V体育ios版)
- VSports手机版 - Actions
- Actions (VSports手机版)
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
V体育2025版 - Other Literature Sources
Research Materials

