Summary
The viral antigen (Ag)-specific CD8+ cytotoxic T lymphocytes (CTLs) derived from pluripotent stem cells (PSCs), i. e VSports注册入口. , PSC-CTLs, have the ability to suppress hepatitis B virus (HBV) infection. After adoptive transfer, PSC-CTLs can infiltrate into the liver to suppress HBV replication. Nevertheless, the mechanisms by which the viral Ag-specific PSC-CTLs provoke the antiviral response remain to be fully elucidated. In this study, we generated the functional HBV surface Ag-specific CTLs from the induced PSC (iPSCs), i. e. , iPSC-CTLs, and investigated the underlying mechanisms of the CTL-mediated antiviral replication in a murine model. We show that adoptive transfer of HBV surface Ag-specific iPSC-CTLs greatly suppressed HBV replication and prevented HBV surface Ag expression. We further demonstrate that the adoptive transfer significantly increased T cell accumulation and production of antiviral cytokines. These results indicate that stem cell-derived viral Ag-specific CTLs can robustly accumulate in the liver and suppress HBV replication through producing antiviral cytokines.
Subject Areas: Immunology, Functional Aspects of Cell Biology
Graphical Abstract

"V体育安卓版" Highlights
-
•
Generation of functional viral Ag-specific CTLs from iPSCs, i. e VSports在线直播. , iPSC-CTLs.
-
•
Viral Ag-specific iPSC-CTLs suppress HBV replication in a murine model
-
•
Adoptive transfer of viral Ag-specific iPSC-CTLs generates persistent α-HBV T cells
-
•
Adoptive transfer of viral Ag-specific iPSC-CTLs produces antiviral IFN-γ and TNF-⍺
Immunology; Functional Aspects of Cell Biology
Introduction
Most acute hepatitis B virus (HBV)-related hepatitis, which is controlled by both humoral and cellular immune responses, develops following acute infection. However, a quantity of individuals in the HBV-endemic areas fail to resolve the infection and consequently become chronic carriers. More than 25% of the chronic patients (>250 million people) worldwide develop progressive liver disease, resulting in liver cirrhosis and/or hepatocellular carcinoma (HCC). Although a vaccine is available and new antiviral drugs are being developed, elimination of persistently infected cells is still a major health issue. Standard treatment for HBV infection includes IFN-α, nucleoside, and nucleotide analogues; these agents have direct antiviral activity and immune modulatory capacities. Nevertheless, seroconversion of HBe antigen (Ag)+ patients to α-HBe antibody (Ab) and loss of serum HBV DNA occur only in about 20% of treated patients, and complete immunological control of the virus evidenced by the loss of the HBs Ag is at best 5% (Gish et al. , 2010). Moreover, response to treatment is often not durable. Prophylactic vaccination with recombinant HBs Ag is highly effective in preventing infection, but therapeutic HBs Ag vaccination is not effective. A robust T cell response is important for control of HBV infection and liver damage; conversely, HBV-specific T cells are usually deleted or dysfunctional or become exhausted in patients with chronic hepatitis (Kurktschiev et al. , 2014; Fisicaro et al. , 2010; Schurich et al V体育官网. , 2013). As a result, efforts to restore virus-specific T cell immunity using antiviral therapy, immunomodulatory cytokines, or therapeutic vaccination have had little success in patients with chronic HBV.
Adoptive cell transfer of cytotoxic T lymphocyte (CTLs) with specificity for HBV Ag+ cells represents an effective approach aiming to ultimately eliminate residual hepatocytes carrying HBV (Gehring et al. , 2011; Xia et al. , 2016; Wisskirchen et al. , 2019; Tan et al. , 2019). Adoptive cell transfer of HBV-specific CTLs into HBV-infected mice has been shown to cause transient, mild hepatitis and a dramatic drop in HBV RNA transcripts in hepatocytes. In these studies, CTLs did not inhibit transcription of HBV genes but enhanced the degradation of HBV transcripts (Huang et al. , 2006). HBV-specific CTLs are important to prevent viral infection and mediate the clearance of HBV (Wong and Pamer, 2003; Murray et al. , 2005). For cell-based therapies, the in vitro generation of highly reactive viral Ag-specific T cells for in vivo re-infusion is considered as an optimal approach (Tan et al. , 2019; Koh et al. , 2018; Hinrichs et al VSports手机版. , 2009, 2011; Kerkar et al. , 2011); yet, the current methodologies can be improved in terms of the capacity to generate, isolate, and expand sufficient quantity and quality of such T cells from patients for therapeutic interventions.
Although clinical trials show safety, feasibility, and potential therapeutic activity of cell-based therapies using engineered T cells with specificity to HBV-infected cells (Koh et al. , 2018; Wisskirchen et al. , 2019; Tan et al. , 2019), there are concerns about the undesirable effects arising from autoimmunity due to cross-reactivity from mispairing TCR (Kuball et al. , 2007; van Loenen et al. , 2010), off-target Ag recognition by non-specific TCR (Cameron et al. , 2013), and on-target off toxicity by chimeric Ag receptor (CAR) (Fedorov et al. , 2013; Maus et al. , 2013) with healthy tissues. Currently, the genetically modified T cells are usually intermediate or later effector T cells, which only have short-term persistence in vivo. To date, pluripotent stem cells (PSCs) are the only source available to generate high numbers of naive single-type Ag-specific T cells (Haque et al. , 2012; Vizcardo et al. , 2013; Nishimura et al. , 2013; Lei et al. , 2011). Induced PSCs (iPSCs) can be easily generated from patients' somatic cells by transduction of various transcription factors and exhibit characteristics identical to those of embryonic stem cells (ESCs) (Kim et al. , 2009) V体育安卓版. Because of the plasticity and the potential for an unlimited capacity for self-renewal, iPSC therapies have great potential in regenerative medicine and tissue replacement. In addition, iPSCs have a high potential to advance the field of cell-based therapies.
Here, we report the development of a robust technique of producing a large amount of viral Ag-specific CTLs from iPSCs, i V体育ios版. e. , iPSC-CTLs that retain all the quintessential characteristics of this T cell subset, including expressions of CD3, CD8, and TCR, and production of cytokines including IFN-γ. We show that adoptive transfer of these viral Ag-specific iPSC-CTLs significantly increased accumulation of CD8+ T cells and produced antiviral cytokines (IFN-γ, TNF-α) in the liver and dramatically reduced the HBV replication in the liver and blood of infected mice. These results demonstrate a great promise of stem cell-derived viral Ag-specific CTLs in the treatment of HBV infections.
Results
Generation of HBV Viral Ag-Specific iPSC-CTLs
We hypothesized that the genetically modified iPSCs with viral Ag-specific TCR, followed by differentiation driven by Notch signaling, would enable the iPSCs to pass hematopoietic and T lineage differentiation checkpoints, resulting in the development of naive single-type viral Ag-specific CD8+ T cells.
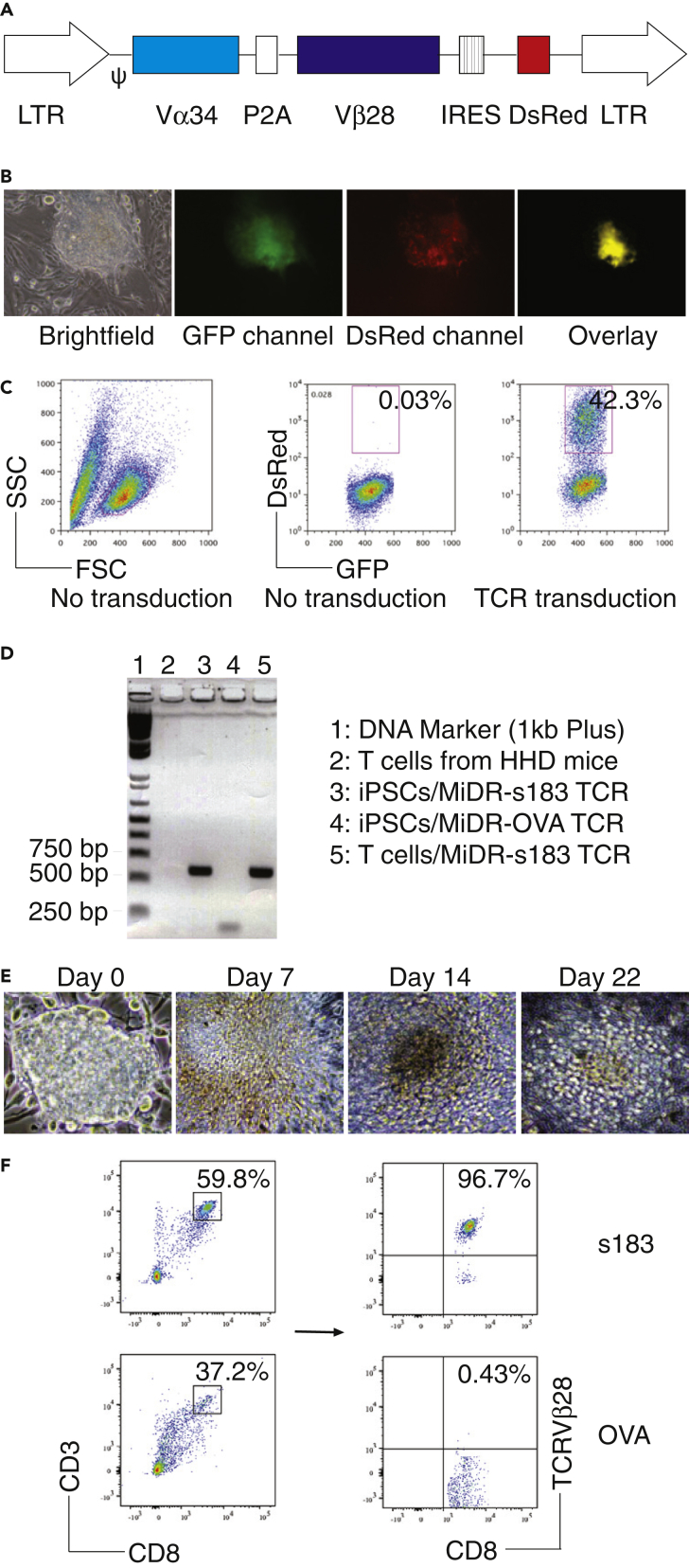
We transduced mouse iPSC (iPS-MEF-Ng-20D-17, GFP+) (Yu et al. , 2009) with the MIDR retroviral construct encoding a human-mouse hybrid HBV TCR gene (HBs183-191-specific—s183; TCR Vα34 and Vβ28) (Figure 1A), or OVA257–264 TCR (MiDR-OVA TCR) (Gehring et al. , 2011) V体育平台登录. We then co-cultured the gene-transduced iPSCs with OP9-DL1/DL4 cells expressing Notch ligands (both DL1 and DL4) molecules in the presence of rFlt3L and rIL-7. Upon gene transduction, we visualized the dsRed expression by fluorescence microscopy (Figure 1B) and sorted GFP+dsRed+ cells (Figure 1C). We confirmed the cloning of HBV TCR into the transduced cells by PCR amplification (Figure 1D) and gene sequencing. After 7 days of culture with the OP9-DL1/DL4 cells, iPSCs differentiated into mesoderm-like cells and showed characteristic, non-adherent grape-like cluster morphology on day 14. On day 22, lymphocyte-like cells spread fully on the culture plates (Figure 1E). On day 28 of in vitro co-culture, the iPSC-derived cells substantially expressed CD3 and Ag-specific TCR, the T cell markers. Flow cytometric analysis of CD3+CD8+ populations showed that the HBV s183 but no OVA TCR transduction dramatically increased the generation of HBV-specific CD8+ T cells (CD8+ TCRVβ28+; Figure 1F). These results suggest that iPSCs have the ability to differentiate into viral Ag-specific CD8+ T cells by the approach of TCR transduction, followed by stimulation with Notch signaling.
Figure 1.
Generation of HBV Viral Ag-Specific iPSC-CTLs
Mouse iPSCs were transduced with the following retroviral constructs: HBs183-91 TCR (MiDR-HBV s183 TCR) or OVA257–264 TCR (MiDR-OVA TCR), and the transduced iPSCs were co-cultured with OP9-DL1/DL4 stromal cells for T lineage differentiation.
(A) Schematic representation of the retrovirus constructs expressing HBV s183 TCR. Ψ, packaging signal; 2A, picornavirus self-cleaving 2A sequence; LTR, Long terminal repeats.
(B) The HBV TCR-transduced iPSCs were visualized by a fluorescence microscope.
(C) GFP+ iPSCs (left and middle, no transduction) were transduced with the retroviral construct MiDR or MiDR with HBV s183 TCR, and the GFP+ dsRed+ iPSCs were analyzed by flow cytometry (right) and sorted by a high-speed cell sorter.
(D) HBV s183 TCR was analyzed for Vβ28 gene expression by PCR. The forward primer is ATGCTGACAGTGCTGCAGGTGCTGCT, and the reverse primer is AGTCGACAACAAGAAGAAGAAGTGGT.
(E) Morphology of T cell differentiation on various days.
(F) Flow cytometric analysis of the iPSC-derived cells on day 28. CD3+CD8+ cells were gated as indicated and analyzed for the expression of CD8 and TCRVβ28. Data shown are representative of three identical experiments.
To determine the functional status of HBV viral Ag-specific iPSC-CTLs, we tested whether these iPSC-CTLs had the capacity to produce the cytokines, following viral Ag stimulation. On day 28 of in vitro co-culture, we isolated the CD4−CD8+ single-positive (SP) iPSC-CTLs and stimulated them by T-depleted splenocytes pulsed with s183 peptide and assessed cytokine production. The iPSC-CTLs produced large amounts of IL-2 and IFN-γ, as detected by intracellular staining (Figure 2A) or ELISA (Figure 2B) and displayed Ag-specific cytotoxicity (Figure 2C), which were similar as HBV TCR gene-transduced CTLs (All p > 0.05; multiple t tests between HBV-specific iPSC-CTLs and HBV-specific CTLs). These results confirmed the generation of functional HBV viral Ag-specific iPSC-CTLs by this approach.
Figure 2.
Functional Analysis of HBV Viral Ag-Specific iPSC-CTLs
On day 28 of in vitro co-culture (described in Figure 1), the SP CD8+s183 TCR pentamer+ iPSC-T cells were sorted. The iPSC-T cells and CD8+ T cells transduced with MiDR-s183 TCR were stimulated by T-depleted splenocytes (APCs) from HHD mice and pulsed with s183 peptide (FLLTRILTI).
(A) Intracellular staining of IL-2 and IFN-γ after 7 h (gated on CD8+ cells) (T/APCs = 1:4).
(B) ELISA of IL-2 and IFN-γ after 40 h. The values represent mean ± SD (∗∗∗∗, p < 0.0001; ns, p > 0.05; ∗∗∗, p <0.001. unpaired t tests).
(C) T cell cytotoxicity was measured after co-culture for 6 h using the 7-AAD/CFSE cell-mediated cytotoxicity assay kit. Data shown are representative of three individual experiments. The values represent mean ± S.D. (∗∗∗∗, p < 0.0001; ns, p > 0.05. Nested one-way ANOVA).
Hydrodynamic Injection Induces HBV Replication in Mice
To demonstrate the protection of HBV infection by adoptive cell transfer of HBV viral Ag-specific iPSC-CTLs, we induced HBV infection in HLA-A2.1 transgenic (HHD) mice by hydrodynamic injection of 10 μg of pAAV/HBV1.2 DNA plasmid through the tail veins of mice. After injection, mice were regularly bled by retroorbital puncture to monitor the serum levels of HBsAg. DNA was isolated from the blood and 100 ng of DNA was used to detect HBV DNA replication using real-time PCR (RT-PCR). We tested various mouse strains for HBV replication using this method as distinct genetic components are known to partially contribute to the outcomes of HBV exposure, in which the Sting-/- mice had a greatest HBV replication (Figure S1). Certain mice in strains C57BL/6, C3H/HeN, and DBA/2 failed to clear HBs Ag within 8 weeks post infection (Chou et al., 2015). We detected HBV replication from day 3 to day 35 in the serum of HHD mice (C57BL/6 background; Figure 3A). DNA replication peaked on day 7 and then reduced gradually. HBV DNA was not cleared from the serum until day 35. We also examined HBs Ag (Figure 3B), HBe Ag (Figure 3C), and alanine aminotransferase (ALT) (Figure 3D) from the serum by ELISA on various days. Similar to DNA replication, HBs Ag peaked on day 7 and then dropped slowly. Conversely, HBe Ag peaked on day 7 but dropped quickly. ALT level was not obvious at all time points, indicating that no substantial liver damage occurred. Intra-hepatic viral DNA transcription and replication were detected from the liver. Mice were sacrificed at different days of post infection, and their livers were isolated and prepared for HBV surface protein staining. In the early period post infection, expression of HBV surface protein was high (approximately 40% of all liver cells are HBs Ag+) and correlates with DNA replication data (Figures 3E and 3F). During the whole period, we detected HBV protein expression following hydrodynamic injection, although the protein expression was lower than that in the initial period.
Figure 3.
Induction of HBV Replication in HHD Mice by Hydrodynamic Injection
HHD mice were i.v. administrated with HBV plasmid via hydrodynamic tail vein injection. Ten micrograms of plasmid dissolved in 8% body weight of PBS was injected.
(A) Serum HBV copies. On indicated time points after injection, the serum was isolated from the blood and DNA was extracted for RT-PCR. Data shown are representative of three individual experiments (n = 5). The line represents mean values.
(B) Serum HBs Ag.
(C) Serum HBe Ag.
(D) Serum ALT. On indicated time points after injection, the serum was isolated from the blood and protein expression was determined by ELISA. Data shown are representative of three individual experiments (n = 5). The lines represent mean values (ns, p > 0.05. Nested one-way ANOVA).
(E) Liver tissue histology. Mice were euthanized on day 8 after HBV infection. Liver samples were isolated and stained for histologic examination. The upper panel shows HBs Ag protein expression (↑) in infected mice (IHC staining) and the lower panel shows the inflammatory cell infiltration (H&E staining). Data are representative of five mice per group of three independent experiments.
(F) Quantitation of HBs Ag+ cells on various days. Data shown are representative of three individual experiments (n = 5). The lines represent mean values (ns, p > 0.05. Nested one-way ANOVA).
We also sought to detect the inflammatory cell infiltration in the liver after viral infection. Liver samples were processed with hematoxylin and eosin (H&E) staining to detect the inflammatory cells. We observed the robust infiltration of inflammatory cells into the liver in the early period followed by gradually reduction (Figure 3E, lower panel). The inflammation persisted until day 35 in all mouse strains. To confirm viral replication, we performed Southern blotting using the DNA from the infected livers. Southern blotting showed the viral DNA replication at all time points (Figure S2). These results are in line with the previous studies demonstrating the hydrodynamic injection-induced HBV replication in mice (Huang et al., 2006).
Accumulation of HBV Viral Ag-Specific iPSC-CTLs in the Mouse Liver with HBV Replication
To exert the cytotoxic effects, HBV viral Ag-specific iPSC-CTLs need to accumulate in the liver in which viral replication initiates and expands, and this requires Ag specificity (Tang et al., 2004). We have previously reported that Ag-specific iPSC-CTLs were detected within the involved tissues, suggesting that these CTLs could traffic in the local tissues at sites of Ag expression (Haque et al., 2016a; Belkaid et al., 2002; Lee et al., 2005).
To accurately express human HBV TCR on mouse iPSCs, we used murine-human hybrid TCR in which the original human constant region was replaced by that of mouse. Also, for the potential recognition of human TCR in mice, we used HHD mice that express human HLA-A2.1 but lack murine major histocompatibility complex (MHC) class I molecules.
Following the hydrodynamic injection of pAAV/HBV1.2 DNA plasmid and adoptive cell transfer of OVA or HBV-specific iPSC-CTLs, we used flow cytometry to analyze the expression of HBV-specific TCR on CD8+ T cells. In the spleens and livers of mice receiving HBV-specific iPSC-CTLs, CD8+ T cells expressing HBV-specific TCR were clearly visualized (3.06/11.87 = 25.8% and 1.74/4.5 = 38.7% of CD8+ populations, respectively) but was barely detected in those mice receiving the control cells, i.e., OVA-specific iPSC-CTLs (Figure 4A). Using immunofluorescence staining, we further visualized HBV-specific TCR expression on CD8+ T cells in the livers but did not detect its expression on those of mice receiving OVA-specific iPSC-CTLs (Figure 4B). These results strongly suggest that HBV viral Ag-specific iPSC-CTLs have the ability to accumulate in the HBV-infected livers.
Figure 4.
Accumulation of HBV Viral Ag-Specific iPSC-CTLs in the Liver with HBV Replication
HHD mice were i.v. administrated with HBV plasmid via hydrodynamic tail vein injection as described in Figure 3, and in the following week were i.v. adoptively transferred with HBs183-91 or OVA257–264-specific dsRed+CD8+ pre-iPSC-CTLs. Mice were euthanized at day 8 after the adoptive transfer of T cells. The spleens or livers were harvested, and the intra-hepatic lymphocytes were isolated by enzymatic digestion.
(A) Accumulation of HBV-specific T cells analyzed by flow cytometry. Single cell suspension was made from the intra-hepatic lymphocytes, and cells were stained with fluorochrome-conjugated antibodies. CD8+ T cells were gated to analyze HBV-specific TCR. Data are representative of five mice per group of three independent experiments.
(B) Immunohistochemistry. Liver slides were stained with both fluorochrome-conjugated CD8 antibody and s183 TCR pentamer and examined under a fluorescent microscope. Green color indicates CD8, and red color indicates HBV-specific TCR. Data are representative of five mice per group of three independent experiments.
HBV Viral Ag-Specific iPSC-CTLs Reduce HBV Replication In Vivo
Next, we tested whether adoptive cell transfer of HBV viral Ag-specific iPSC-CTLs could prevent or reduce viral replication. Mice were intravenously (i.v.) administrated pAAV/HBV1.2 DNA plasmid via hydrodynamic tail vein injection, and in the following week were i.v. adoptively transferred with HBs183-91 or OVA257–264-specific dsRed+CD8+ iPSC-CTLs. The viral titer was measured by RT-PCR from the serum at various time points after the adoptive transfer of T cells. The results demonstrated that viral replication was significantly reduced at all time points in the mice receiving HBV viral Ag-specific T cells as compared with the mice receiving the control OVA-specific iPSC-CTLs (Figure 5A). In these adoptive cell transfer-based regimens, all groups of mice had similar serum ALT profiles, indicating that there were no obvious liver damages after adoptive cell transfer of HBV-specific CTLs (Figure 5B). We also examined the HBV surface protein expression in the livers in the above setting of treatment. Mice were euthanized at various days after the HBV injection, and the liver samples were isolated for histologic examination. Samples were stained for HBV surface protein and examined under a microscope. We observed that HBV surface protein was substantially decreased in the mice receiving HBV viral Ag-specific iPSC-CTLs, as compared with the mice receiving control cells (Figures 5C and 5D), and this is associated with inflammatory cell infiltration visualized by H&E staining. Collectively, these results suggest that adoptive transfer of HBV viral Ag-specific iPSC-CTLs have the ability to reduce HBV replication at both DNA and protein levels.
Figure 5.
In Vivo Reduction of HBV Replication by Viral Ag-Specific iPSC-CTLs
HHD mice were i.v. administrated with HBV plasmid via hydrodynamic tail vein injection, and in the following week were i.v. adoptively transferred with HBs183-91 or OVA257–264-specific dsRed+CD8+ iPSC-CTLs.
(A) Serum HBV copies. At the indicated time points after the adoptive transfer of T cells, serum was isolated from the blood and DNA was extracted for RT-PCR analysis. Data shown are three individual experiments (n = 5). The lines represent mean values from the three experiments (∗∗, p < 0.01. Nested one-way ANOVA).
(B) Serum ALT. Data shown are three individual experiments (n = 5) (ns, p > 0.05. Nested one-way ANOVA).
(C) Liver tissue histology. Mice were euthanized on day 8 after the adoptive transfer of T cells. Liver samples were isolated and stained for histologic examination. The upper panel shows HBs Ag protein expression (↑) in infected mice (IHC staining), and the lower panel shows the inflammatory cell infiltration (H&E staining). Data are representative of five mice per group of three independent experiments.
(D) Quantitation of HBs Ag-positive cells at various days. Data shown are three individual experiments (n = 5). The lines represent mean values from the three experiments (∗∗∗∗, p < 0.0001. Nested t test).
HBV Viral Ag-Specific iPSC-CTLs-Induced Reduction of HBV Replication Is Mediated by IFN-γ and TNF-α
The clearance of intracellular pathogens by the immune response is widely believed to be mediated primarily by the destruction of infected cells by major histocompatibility complex (MHC) class-I restricted CD8+ T cells. Upon Ag recognition, CTLs can secrete potent antiviral cytokines such as IFN-γ and TNF-α, which can effectively eliminate viral infection without killing the infected cells (Khakpoor et al., 2019). To evaluate the extent to which the antiviral effects reduce HBV replication, we examined the production of the antiviral cytokines IFN-γ and TNF-α from the intra-hepatic lymphocytes of mice receiving HBV viral Ag-specific or OVA-specific iPSC-CTLs. Intra-hepatic lymphocytes were isolated from both groups of mice and stained for IFN-γ and TNF-α. CD8+ T cells were gated, and their productions of IFN-γ and TNF-α were determined by flow cytometry. The number of IFN-γ-producing CD8+ T cells in the livers was considerably higher in mice receiving HBV viral Ag-specific iPSC-CTLs than in mice receiving the control OVA-specific cells (3.89/22.69 = 17.1% versus <1%; Figures 6A and 6C, upper panel). A similar observation was obtained in the number of TNF-α-producing CD8+ T cells (11.85/29.75 = 37.3% versus <1%; Figures 6A and 6C, lower panel). Furthermore, we prepared the liver samples for immunofluorescence staining for detection of IFN-γ or TNF-α-producing CD8+ T cells. More IFN-γ or TNF-α-producing CD8+ T cells were observed in the livers of mice receiving HBV viral Ag-specific iPSC-CTLs than that of mice receiving OVA-specific cells (Figure 6B). In addition, flow cytometric analysis showed approximately 16.8% of CD8+ T cells producing both IFN-γ and TNF-α in mice receiving HBV viral Ag-specific iPSC-CTLs (Figure 6D).
Figure 6.
Critical Role of IFN-γ and TNF-α by Viral Ag-Specific iPSC-CTLs in the Reduction of HBV Replication
HHD mice were i.v. administrated with HBV plasmid via hydrodynamic tail vein injection, and in the following week were i.v. adoptively transferred with HBs183-91 or OVA257–264-specific dsRed+CD8+ iPSC-CTLs. In some experiments, the livers were harvested, and the intra-hepatic lymphocytes were isolated by enzymatic digestion (A–D). In other experiments (E–G), after the cell transfer, mice were i.p. injected α-mouse IFN-γ, TNF-α, IFN-γ with TNF-α, or rat IgG1 control (10 mg/kg, three times in the first week after the cell transfer). Serum HBV copies on indicated time points after the administration of HBV plasmid were determined by RT-PCR.
(A) IFN-γ or TNF-α-producing CD8+ T cells analyzed by intracellular staining and flow cytometric analysis. Data are representative of five mice per group of three independent experiments.
(B) Immunohistochemistry. Liver slides were stained with fluorescence-conjugated CD8 and IFN-γ or TNF-α antibodies and examined under a fluorescent microscope (Red: CD8+; Green: IFN-γ+ or TNF-α+). Data are representative of five mice per group of three independent experiments.
(C) Percentage of IFN-γ or TNF-α-producing CD8+ T cells. Data shown are three individual experiments (n = 5). The values represent mean ± SEM (∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, Unpaired t test).
(D) IFN-γ and TNF-α-producing CD8+ T cells analyzed by intracellular staining and flow cytometric analysis, gating on CD8+ populations. Data are representative of five mice per group of three independent experiments.
(E) α-IFN-γ.
(F) α-TNF-α.
(G) α-IFN-γ with anti-TNF-α. Data shown are three individual experiments (n = 5). The lines represent mean values from the three experiments (∗, p < 0.05; ∗∗, p < 0.01. Nested t test).
To further validate the critical roles of IFN-γ and TNF-α that were produced from HBV-specific iPSC-CTLs were responsible for the reduction of HBV replication, we i.p. injected mice with neutralizing antibodies to IFN-γ or TNF-α alone or both. At all time points, either neutralizing antibody to IFN-γ or TNF-α could dramatically increase serum HBV DNA copies (p < 0.05) (Figures 6E and 6F), and the administration of both neutralizing antibodies at the time points after 1 week significantly augmented serum HBV DNA copies (p < 0.01) (Figure 6G). Collectively, these results suggest that the increased production of antiviral cytokines IFN-γ and TNF-α is responsible for the reduction of HBV replication.
"V体育安卓版" HBV Viral Ag-Specific iPSC-CTLs Persist In Vivo
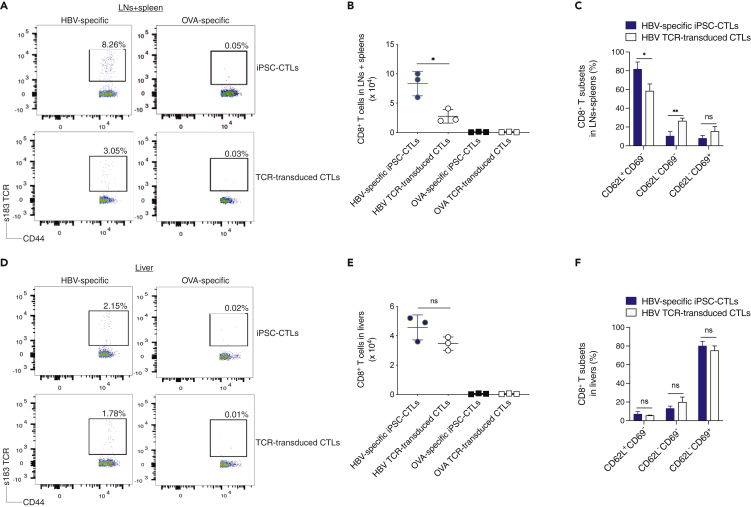
The naive or central memory T cell-derived effector CTLs, known as “highly reactive” cells, are considered as the optimal populations for adoptive cell transfer-based therapies, as these cells have high proliferative potential, are less prone to apoptosis than terminally differentiated cells, and have higher ability to respond to homeostatic cytokines such as IL-7 and IL-15 (Klebanoff et al., 2016). We next tested whether the adoptive transfer using the HBV viral Ag-specific iPSC-CTLs could generate T cell persistence that is critical for protecting against HBV infection. We hydrodynamically injected HHD mice with the pAAV/HBV1.2 DNA plasmid and in the following week performed the adoptive transfer using HBs183-91 or OVA257-264 TCR gene-transduced CD8+ T cells from the lymph nodes (LNs) and spleen of HHD mice or the Ag (HBV or OVA)-specific mouse iPSC-CTLs into HHD mice. Thirty-five days later, the T cell persistence was analyzed by tracking CD8+CD44+ s183 TCR+ cells from the pooled LNs and spleen or the liver. A greater number of CD44+ s183 TCR+ persistent CD8+ T cells developed in mice receiving HBV-specific iPSC-CTLs than in the animals receiving HBV TCR-transduced T cells (8.26% versus 3.05%) in the pooled LNs and spleen, as analyzed by flow cytometric analysis (Figure 7A) and cell number (Figure 7B; p < 0.05), but not in the liver (Figures 7D and 7E). We further analyzed the various T cell subsets, i.e., CD62L+CD69−, CD62L−CD69−, and CD62L−CD69+, gating on CD44hi s183 TCR+ populations. In the pooled LNs and spleen, HBV-specific iPSC-CTLs developed more CD62L+CD69− cells but fewer CD62L−CD69− cells than TCR-transduced T cells (Figure 7C; p < 0.05 or p < 0.01). In the liver, the two groups had no significant difference (Figure 7F; p > 0.05). The control transfers with equal number of OVA-specific iPSC-CTLs or OVA TCR-transduced CTLs did not generate any obvious HBV-specific persistent T cells in the LNs and spleen (Figures 7A and 7B) or the liver (Figures 7D and 7E). These results indicate that HBV viral Ag-specific iPSC-CTLs have the ability to generate T cell persistence.
Figure 7.
HBV Viral Ag-Specific iPSC-CTLs Persist In Vivo
HHD mice were i.v. administrated with HBV plasmid via hydrodynamic tail vein injection and, in the following week, were i.v. adoptively transferred with Ag (HBs183-91 or OVA257-264)-specific iPSC-CTLs or the Ag TCR gene-transduced CD8+ T cells from HHD mice.
(A–F) (A) At day 35 after the injection of HBV plasmid, the pooled LNs and spleen (A–C) or the liver (D–F) were analyzed for the T cell persistence. (A and D) Persistent T cells by flow cytometry using CD44 and HBV s183 TCR pentamer staining, gating on CD8+ populations. (B and E) Number quantification of persistent CD8+ T cell populations. (C and F) Quantification of various persistent CD8+ T cell subsets. Data shown are the representative of three identical experiments. The values represent mean ± SD (∗p < 0.05, Unpaired t test).
VSports最新版本 - Discussion
T lymphocytes (or T cells) are an essential component of normal immune surveillance systems, and their dysfunction leads to the development of fatal diseases such as cancers (e.g., liver cancer) and autoimmune diseases (e.g., systemic lupus erythematous). Under the appropriate circumstance, PSCs can produce almost all types of cells in the body, including T cells. Thus, PSCs provide a chance to obtain a renewable source of healthy T cells for treating a wide array of diseases. However, the optimal circumstance for development of T cells from PSCs (i.e., PSC-T cells) has not been fully defined. We have previously reported the development of tumor Ag-specific iPSC-CTLs and use of these CTLs as an adoptive cell transfer for suppressing tumor growth in an animal model (Lei et al., 2011, 2017). In the current study, we developed viral Ag-specific iPSC-CTLs and used them as adoptive cell transfer to reduce HBV replication in a murine model. We further showed that the viral Ag-specific iPSC-CTLs accumulate in the liver and mediate the reduction of HBV replication through the production of large amounts of IFN-γ and TNF-α. Furthermore, we showed that the adoptive cell transfer using viral Ag-specific iPSC-CTLs generated T cell persistence. These findings may help better understand HBV pathogenesis and provide a groundwork for therapeutic use of stem cell-derived viral Ag-specific CTLs in the treatment of HBV infections and HBV-associated liver cancer.
In chronic HBV infection, the viral genome forms a stable mini-chromosome, the covalently closed circular DNA (cccDNA) that can persist throughout the lifespan of the hepatocyte. Targeting the clearance of the viral mini-chromosome may result in a cure of chronic HBV infection. Current antiviral therapy targets the viral reverse transcriptase but rarely establishes immunological control over HBV replication driven by cccDNA. HBV-specific CD8+ CTLs can mediate the killing of the infected hepatocytes and accelerate the clearance of cccDNA. Nevertheless, the HBV-specific CTLs can be deleted or dysfunctional or succumb to exhaustion in patients with chronic HBV infection (Benechet and Iannacone, 2017; Kawashima et al., 2018). Moreover, recent studies showed that priming by hepatocytes, CD8+ T cells differentiated into dysfunctional HBV-specific CTLs, with partial overlap with those of exhausted or tolerant T cells; thus, these HBV-specific CTLs could not be rescued by treatment with immune checkpoint inhibitors such as anti-PD-L1 or CD40-mediated myeloid dendritic cells (mDCs)-activation (Benechet et al., 2019; Isogawa et al., 2013). Adoptive cell transfer of the HBV-specific CTLs has been considered as a highly promising treatment for chronic HBV infection (Tan et al., 2019; Wu et al., 2019). We previously showed that reprogramming of Ag-specific CTLs or regulatory T cells from iPSCs can be used for cell-based therapies (Haque et al., 2012, 2016a, 2016b, 2019; Lei et al., 2011). In this study, we developed a system to generate HBV-specific iPSC-CTLs and provide new insights into the methodologies and mechanistic requirements for efficient development of viral Ag-specific PSC-CTLs. Especially, after adoptive transfer, such PSC-CTLs may overcome exhausted immune cell phenotypes and develop durable anti-HBV immunity.
Furthermore, we found that IFN-γ and TNF-α produced by HBV viral Ag-specific iPSC-CTLs accumulated in the liver and mediated the reduction of HBV replication at both DNA and protein levels. The clearance of intracellular pathogens by the immune response is widely thought to be mediated primarily through the destruction of the infected cells by MHC class I-restricted CD8+ CTLs. Upon Ag recognition, CTLs can also secrete potent antiviral cytokines such as IFN-γ and TNF-α (Khakpoor et al., 2019; Zeng et al., 2016; Xia et al., 2016; Park et al., 2016), which can eradicate viral infections without killing the infected cells. Thus, we examined the productions of antiviral cytokines in the liver and revealed that HBV viral Ag-specific iPSC-CTLs-induced reduction of HBV replication was mediated by IFN-γ and TNF-α. Of note, we did not detect the obvious destruction of the hepatocytes (Figure 5B), which might be associated with HBV Ag presentation. It has been suggested that, by priming by Kupffer cells, but not hepatocytes, viral Ag-specific CD8+ T cells could efficiently differentiate into functional effector cells against HBV infection (Benechet et al., 2019). In vitro, HBV viral Ag-specific iPSC-CTLs showed the ability to induce specified lysis of target cells, in an effector-to-target ratio-specific manner (Figure 2C), which may not represent in a physiological condition in vivo. Nevertheless, adoptive cell transfer of HBV viral Ag-specific iPSC-CTLs may eliminate HBV replication mainly through secreting antiviral cytokines without killing the hepatocytes, which is in line with a previous observation (Wu et al., 2019).
Although there are transgenic mouse models of HBV replication, these models are challenging because the central tolerance induced by the transgenic gene products causes mice to be immune tolerant to HBV Ags. Additionally, transgenic mice are not suitable for monitoring viral clearance as the integrated HBV genome persists in each mouse cell (Chisari et al., 1987; Wirth et al., 1995). Also, although successful vaccines have been developed for preventing the infection, the treatment or immunotherapy after HBV infection has not been developed. Furthermore, the experimental approaches to HBV pathogenesis have been hampered because the host range of HBV infection is limited to human beings and chimpanzees, and in vitro culture system for the propagation of HBV is not sufficient. CD8+ T cells are promising effector cells against various types of viral infection; however, T cell response against HBV is not abundant. Specificity, functioning, and lack of sufficient amount to mount an immune response may be the cause. Here, we used the method of hydrodynamic injection that capably induces HBV replication in mice. The method allows delivery of a large amount of HBV plasmid directly into the liver. The model exhibits continuous viremia for more than 8 weeks with detection of HBV mRNA, protein, and DNA at different days after injection. This method for HBV replication in mice may be a useful model for HBV immunotherapy. Of note, this method brought out a large number of infiltrating immune cells in the liver tissues (Figures 3E and 5C), and we identified that the majority of these inflammatory immune cells were CD11b+Ly6G+ neutrophils (Figure S3).
Taken together, the current study provides new insights into the mechanism and therapeutic intervention using viral Ag-specific iPSC-CTLs as immunotherapy for HBV infections. However, in the current study, the HBV hydrodynamic scheme is not a real model of chronic HBV infection, but it is a method to express HBV Ags in mouse hepatocytes. The robust mouse models for studying HBV persistence and therapeutic intervention may be the adenovirus and adeno-associated virus (AAV)-based systems (Dion et al., 2013; Sandhu et al., 2017), which mimic chronic HBV infection. However, there are still disadvantages in the mouse models in which HBV viruses are transient replication in the liver, and no real HBV viral infection as well as cccDNA formation. Nevertheless, a combination approach using α-HBV drugs with adoptive cell transfer of viral Ag-specific iPSC-CTLs is likely to reduce HBV reservoirs, thereby resulting in a remedy of HBV infections.
Limitations of the Study
The in vitro generation of viral Ag-specific T lymphocytes from stem cells has not been optimized, and the iPSC-derived CD8+ T cells did not differentiate at the same time. We sorted dsRed+CD8+ cells for adoptive cell transfer, and the dsRed+CD8+ cells might include CD4+CD8+ (DP) cells and other CD8+ premature lymphocytes. After the adoptive cell transfer, the premature lymphocytes might change CD8 expression.
The robust mouse models for studying HBV persistence and therapeutic intervention may be the adenovirus and adeno-associated virus (AAV)-based systems, which mimic chronic HBV infection. However, there are still disadvantages in the mouse models in which HBV viruses are transient replication in the liver, and no real HBV viral infection as well as cccDNA formation.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jianxun Song (jus35@qiuluzeuv.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplemental Information.
Methods (V体育2025版)
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors thank Dr. Pei-Jer Chen from National Taiwan University Hospital for providing the pAAV/HBV1.2 construct and Dr. Adam J Gehring from Toronto General Hospital Research Institute for providing the s183-specific TCR genes. The authors also would like to acknowledge the critical review of the manuscript by Dr. Jianming Hu from The Pennsylvania State University College of Medicine.
Guarantor's statement: J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding/financial support: This work was supported by the National Institutes of Health Grant R01AI121180 and R21AI128325 to J.S. and R01CA221867 to J.-M.Y. and J.S.
Author Contributions
J.S. and J.-M.Y. designed the experiments, analyzed data, and contributed to the writing of the paper. M.H., F.L., Y.R., A.K., and J.K.D. performed the experiments. D.F. and P.d.F. provided reagents for the experiments. X.X. and X.R. analyzed data.
Declaration of Interests
The authors have declared that no conflict of interest exists.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101333.
Supplemental Information
References
- Belkaid Y., Piccirillo C.A., Mendez S., Shevach E.M., Sacks D.L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Benechet A.P., de Simone G., di Lucia P., Cilenti F., Barbiera G., le Bert N., Fumagalli V., Lusito E., moalli F., Bianchessi V. Dynamics and genomic landscape of CD8(+) T cells undergoing hepatic priming. Nature. 2019;574:200–205. doi: 10.1038/s41586-019-1620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benechet A.P., Iannacone M. Determinants of hepatic effector CD8(+) T cell dynamics. J. Hepatol. 2017;66:228–233. doi: 10.1016/j.jhep.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Cameron B.J., Gerry A.B., Dukes J., Harper J.V., Kannan V., Bianchi F.C., GRAND F., brewer J.E., Gupta M., Plesa G. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F.V., Filippi P., Buras J., McLachlan A., Popper H., Pinkert C.A., Palmiter R.D., Brinster R.L. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc. Natl. Acad. Sci. U S A. 1987;84:6909–6913. doi: 10.1073/pnas.84.19.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou H.H., Chien W.H., Wu L.L., Cheng C.H., Chung C.H., Horng J.H., Ni Y.H., Tseng H.T., Wu D., Lu X. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc. Natl. Acad. Sci. U S A. 2015;112:2175–2180. doi: 10.1073/pnas.1424775112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion S., Bourgine M., Godon O., Levillayer F., Michel M.L. Adeno-associated virus-mediated gene transfer leads to persistent hepatitis B virus replication in mice expressing HLA-A2 and HLA-DR1 molecules. J. Virol. 2013;87:5554–5563. doi: 10.1128/JVI.03134-12. [DOI (V体育安卓版)] [PMC free article] [PubMed] [Google Scholar]
- Fedorov V.D., Themeli M., Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI (V体育官网入口)] [PMC free article] [PubMed] [Google Scholar]
- Fisicaro P., Valdatta C., Massari M., Loggi E., Biasini E., Sacchelli L., Cavallo M.C., Silini E.M., Andreone P., Missale G., Ferrari C. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682–693. doi: 10.1053/j.gastro.2009.09.052. 693.e1-4. [DOI] [PubMed] [Google Scholar]
- Gehring A.J., Xue S.A., Ho Z.Z., Teoh D., Ruedl C., Chia A., Koh S., Lim S.G., Maini M.K., Stauss H., Bertoletti A. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J. Hepatol. 2011;55:103–110. doi: 10.1016/j.jhep.2010.10.025. [V体育官网 - DOI] [PubMed] [Google Scholar]
- Gish R.G., Chang T.T., Lai C.L., de Man R., Gadano A., Poordad F., Yang J., Brett-Smith H., Tamez R. Loss of HBsAg antigen during treatment with entecavir or lamivudine in nucleoside-naive HBeAg-positive patients with chronic hepatitis B. J. Viral Hepat. 2010;17:16–22. doi: 10.1111/j.1365-2893.2009.01146.x. ["V体育ios版" DOI] [PubMed] [Google Scholar]
- Haque R., Lei F., Xiong X., Bian Y., Zhao B., Wu Y., Song J. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J. Immunol. 2012;189:1228–1236. doi: 10.4049/jimmunol.1200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M., Song J., Fino K., Sandhu P., Song X., Lei F., Zheng S., Ni B., Fang D., Song J. Stem cell-derived tissue-associated regulatory T cells ameliorate the development of autoimmunity. Sci. Rep. 2016;6:20588. doi: 10.1038/srep20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M., Song J., Fino K., Sandhu P., Wang Y., Ni B., Fang D., Song J. Melanoma immunotherapy in mice using genetically engineered pluripotent stem cells. Cell Transplant. 2016;25:811–827. doi: 10.3727/096368916X690467. ["V体育安卓版" DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M., Lei F., Xiong X., Das J.K., Ren X., Fang D., Salek-Ardakani S., Yang J.M., Song J. Stem cell-derived tissue-associated regulatory T cells suppress the activity of pathogenic cells in autoimmune diabetes. JCI Insight. 2019;4:e126471. doi: 10.1172/jci.insight.126471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs C.S., Borman Z.A., Cassard L., Gattinoni L., Spolski R., Yu Z., Sanchez-Perez L., Muranski P., Kern S.J., Logun C. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. U S A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. ["V体育安卓版" DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs C.S., Borman Z.A., Gattinoni L., Yu Z., Burns W.R., Huang J., Klebanoff C.A., Johnson L.A., Kerkar S.P., Yang S. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.R., Wu H.L., Chen P.J., Chen D.S. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. U S A. 2006;103:17862–17867. doi: 10.1073/pnas.0608578103. ["VSports注册入口" DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogawa M., Chung J., Murata Y., Kakimi K., Chisari F.V. CD40 activation rescues antiviral CD8(+) T cells from PD-1-mediated exhaustion. PLoS Pathog. 2013;9:e1003490. doi: 10.1371/journal.ppat.1003490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima K., Isogawa M., Hamada-Tsutsumi S., Baudi I., Saito S., Nakajima A., Tanaka Y. Type I interferon signaling prevents hepatitis B virus-specific T cell responses by reducing antigen expression. J. Virol. 2018;92:e01099-18. doi: 10.1128/JVI.01099-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar S.P., Sanchez-Perez L., Yang S., Borman Z.A., Muranski P., JI Y., Chinnasamy D., Kaiser A.D., Hinrichs C.S., Klebanoff C.A. Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies. J. Immunother. 2011;34:343–352. doi: 10.1097/CJI.0b013e3182187600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpoor A., Ni Y., Chen A., Ho Z.Z., Oei V., Yang N., Giri R., Chow J.X., Tan A.T., Kennedy P.T. Spatiotemporal differences in presentation of CD8 T cell epitopes during hepatitis B virus infection. J. Virol. 2019;93:e01457-18. doi: 10.1128/JVI.01457-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.B., Sebastiano V., Wu G., Arauzo-Bravo M.J., Sasse P., Gentile L., Ko K., Ruau D., Ehrich M., van den Boom D. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Klebanoff C.A., Scott c.D., Leonardi A.J., Yamamoto T.N., Cruz A.C., Ouyang C., Ramaswamy M., Roychoudhuri R., JI Y., Eil R.L. Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J. Clin. Invest. 2016;126:318–334. doi: 10.1172/JCI81217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S., Kah J., Tham C.Y.L., Yang N., Ceccarello E., Chia A., Chen M., Khakpoor A., Pavesi A., Tan A.T. Nonlytic lymphocytes engineered to express virus-specific T-cell receptors limit HBV infection by activating APOBEC3. Gastroenterology. 2018;155:180–193.e6. doi: 10.1053/j.gastro.2018.03.027. [DOI] [PubMed] [Google Scholar]
- Kuball J., Dossett M.L., Wolfl M., Ho W.Y., Voss R.H., Fowler C., greenberg P.D. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurktschiev P.D., Raziorrouh B., Schraut W., Backmund M., Wachtler M., Wendtner C.M., Bengsch B., Thimme R., Denk G., Zachoval R. Dysfunctional CD8+ T cells in hepatitis B and C are characterized by a lack of antigen-specific T-bet induction. J. Exp. Med. 2014;211:2047–2059. doi: 10.1084/jem.20131333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Wang L., Wells A.D., Dorf M.E., Ozkaynak E., Hancock W.W. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei F., Zhao B., Haque R., Xiong X., Budgeon L., Christensen N.D., Wu Y., Song J. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res. 2011;71:4742–4747. doi: 10.1158/0008-5472.CAN-11-0359. [DOI] [PubMed] [Google Scholar]
- Lei F., Haque M., Sandhu P., Ravi S., Song J., Ni B., Zheng S., Fang D., Jia H., Yang J.M., Song J. Development and characterization of naive single-type tumor antigen-specific CD8(+) T lymphocytes from murine pluripotent stem cells. Oncoimmunology. 2017;6:e1334027. doi: 10.1080/2162402X.2017.1334027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus M.V., Haas A.R., Beatty G.L., Albelda S.M., Levine B.L., Liu X., Zhao Y., Kalos M., June C.H. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.M., Wieland S.F., Purcell R.H., Chisari F.V. Dynamics of hepatitis B virus clearance in chimpanzees. Proc. Natl. Acad. Sci. U S A. 2005;102:17780–17785. doi: 10.1073/pnas.0508913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Kaneko S., Kawana-Tachikawa A., Tajima Y., Goto H., Zhu D., Nakayama-Hosoya K., Iriguchi S., Uemura Y., Shimizu T. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Park Y.K., Park E.S., Kim D.H., Ahn S.H., Park S.H., Lee A.R., Park S., Kang H.S., Lee J.H., Kim J.M. Cleaved c-FLIP mediates the antiviral effect of TNF-alpha against hepatitis B virus by dysregulating hepatocyte nuclear factors. J. Hepatol. 2016;64:268–277. doi: 10.1016/j.jhep.2015.09.012. [DOI (V体育平台登录)] [PubMed] [Google Scholar]
- Sandhu P., Haque M., Humphries-Bickley T., Ravi S., Song J. Hepatitis B virus immunopathology, model systems, and current therapies. Front. Immunol. 2017;8:436. doi: 10.3389/fimmu.2017.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurich A., Pallett L.J., Lubowiecki M., Singh H.D., Gill U.S., Kennedy P.T., Nastouli E., Tanwar S., Rosenberg W., Maini M.K. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013;9:e1003208. doi: 10.1371/journal.ppat.1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A.T., Yang N., Lee Krishnamoorthy T., Oei V., Chua A., Zhao X., Tan H.S., Chia A., Le Bert N., Low D. Use of expression profiles of HBV-DNA integrated into genomes of hepatocellular carcinoma cells to select T cells for immunotherapy. Gastroenterology. 2019;156:1862–1876.e9. doi: 10.1053/j.gastro.2019.01.251. [DOI] [PubMed] [Google Scholar]
- Tang Q., Henriksen K.J., Bi M., Finger E.B., Szot G., Ye J., Masteller E.L., McDevitt H., Bonyhadi M., Bluestone J.A. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loenen M.M., de Boer R., Amir A.L., Hagedoorn R.S., Volbeda G.L., Willemze R., van Rood J.J., Falkenburg J.H., Heemskerk M.H. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc. Natl. Acad. Sci. U S A. 2010;107:10972–10977. doi: 10.1073/pnas.1005802107. [DOI (VSports最新版本)] [PMC free article] [PubMed] [Google Scholar]
- Vizcardo R., Masuda K., Yamada D., Ikawa T., shimizu K., Fujii S., Koseki H., Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Wirth S., Guidotti L.G., Ando K., Schlicht H.J., Chisari F.V. Breaking tolerance leads to autoantibody production but not autoimmune liver disease in hepatitis B virus envelope transgenic mice. J. Immunol. 1995;154:2504–2515. [PubMed] [Google Scholar]
- Wisskirchen K., Kah J., Malo A., Asen T., Volz T., Allweiss L., Wettengel J.M., Lutgehetmann M., Urban S., Bauer T. T cell receptor grafting allows virological control of Hepatitis B virus infection. J. Clin. Invest. 2019;129:2932–2945. doi: 10.1172/JCI120228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P., Pamer E.G. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- Wu L.L., Peng W.H., Wu H.L., Miaw S.C., Yeh S.H., Yang H.C., Liao P.H., Lin J.S., Chen Y.R., Hong Y.T. Lymphocyte antigen 6 complex, locus C(+) monocytes and Kupffer cells orchestrate liver immune responses against hepatitis B virus in mice. Hepatology. 2019;69:2364–2380. doi: 10.1002/hep.30510. [DOI] [PubMed] [Google Scholar]
- Xia Y., Stadler D., Lucifora J., Reisinger F., Webb D., Hosel M., Michler T., Wisskirchen K., Cheng X., Zhang K. Interferon-gamma and tumor necrosis factor-alpha produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology. 2016;150:194–205. doi: 10.1053/j.gastro.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Yu J., Hu K., Smuga-Otto K., Tian S., Stewart R., Slukvin I.I., Thomson J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Li L., Chen Y., Wei H., Sun R., Tian Z. Interferon-gamma facilitates hepatic antiviral T cell retention for the maintenance of liver-induced systemic tolerance. J. Exp. Med. 2016;213:1079–1093. doi: 10.1084/jem.20151218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
"V体育平台登录" Supplementary Materials
Data Availability Statement (V体育安卓版)
The authors confirm that the data supporting the findings of this study are available within the article and its Supplemental Information.