Abstract
Ca2+-activated K+ (KCa)channels are a unique family of ion channels because they are capable of directly communicating calcium signals to changes in cell membrane potential required for cellular processes including but not limited to cellular proliferation and migration. It is now possible to distinguish three families of KCa channels based on differences in their biophysical and pharmacological properties as well as genomic sequence VSports最新版本. Using a combination of biochemical, molecular, and biophysical approaches, we show that human tumor cells of astrocytic origin, i. e. glioma cells, express transcripts for all three family members of KCa channels including BK, IK, and all three SK channel types (SK1, SK2, and SK3). The use of selective pharmacological inhibitors shows prominent expression of currents that are inhibited by the BK channel specific inhibitors iberiotoxin and paxilline. However, despite the presence of transcripts for IK and SK, neither clotrimazole, an inhibitor of IK channels, nor apamin, known to block most SK channels inhibited any current. The exclusive expression of functional BK channels was further substantiated by shRNA knockdown experiments, which selectively reduced iberiotoxin sensitive currents. Western blotting of patient biopsies with antibodies specific for all three KCa channel types further substantiated the exclusive expression of BK type KCa channels in vivo. This finding is in sharp contrast to other cancers that express primarily IK channels.
Keywords: BK channel, migration, astrocyte, glia
INTRODUCTION
K+ channels establish and maintain the resting potential of most living cells V体育平台登录. Their activity is predominantly regulated by the membrane voltage or the K+ gradient across the cell membrane. However, many cells also express Ca2+-activated potassium (KCa) channels, which have the unique ability to translate changes in the level of the intracellular second messenger, Ca2+, to changes in membrane K+ conductance and, hence, resting membrane potential (Stocker, 2004; Swarthout and Walling, 2000).
KCa channels come in three principle varieties that differ with regards to their biophysical and pharmacological features. Small conductance or SKCa channels derive from three separate genes (KCa2. 1, KCa2. 2, and KCa2. 3) that belong to the KCNN gene family (Stocker, 2004). They are predominantly expressed in the nervous system and are voltage insensitive, i. e. are gated only by rises in intracellular Ca2+. They have a relatively small conductance of 5-10 pS and are typically blocked by the bee venom toxin apamin. Intermediate conductance or IKCa channels derive from a closely related gene (KCa3. 1) that gives rise to channels of ∼20-60 pS conductance, i. e VSports注册入口. a value intermediate to small (SK) and big conductance (BK) channels. These channels are sensitive to the antifungal drug clotrimazole and are mainly found in blood and epithelial cells and some peripheral neurons (Jensen et al. , 2002; Stocker, 2004). IK channels are believed to be the “Gardos” channel involved in the dehydration of red blood cells in patients with sickle cell anemia (Maher and Kuchel, 2003). The most diverse group of KCa channels are the big conductance or BKCa family of channels, which are often called “MaxiK+ channels” with conductances in excess of 100 pS (Barrett et al. , 1982; Blatz and Magleby, 1987). Twelve distinct members of this group have now been identified, which are derived from a single gene, the human equivalent of the Drosophila slowpoke “Slo” gene, by alternative splicing (Ferrer et al. , 1996; Liu et al. , 2002; Tseng-Crank et al. , 1994). Most of these splice variants are sensitive to iberiotoxin, charybdotoxin, and paxilline and often associate with 1 of 4 different β subunits that alter their biophysical and pharmacological properties (Orio et al. , 2002).
While all three classes of KCa channels share their regulation by intracellular Ca2+, they are otherwise rather distinct entities with different tissue distribution and presumed function. BKCa channels are known for their ability to regulate vascular tone of blood vessels (Sah and Davies, 2000; Sah and Louise Faber, 2002). SK channels are widely expressed in the nervous system where they are involved in regulating the firing frequency of many neurons including nociceptive neurons (Bahia et al. , 2005). IKCa channels on the other hand are thought to be absent in normal brain but have been described in numerous cancer cells where they have been implicated in growth control V体育官网入口.
In light of the well documented role that Ca2+ signaling plays in glial cells (for review see (Charles, 1998)), it is surprising that we know relatively little about the expression and function of KCa channels in glial cells. MacVicar and colleagues showed small conductance channels in cell attached patches of astrocytes from rat cortex that were sensitive to changes in Ca2+ and blocked by TEA (Quandt and MacVicar, 1986). Similarly, Nowak and colleagues described a 230 pS, Ca2+ sensitive BK channel in inside-out patches from mouse cortical astrocytes (Nowak et al. , 1987). O4+ oligodendrocyte progenitor cells (OPCs) transiently express apamin-sensitive SK channels, which disappear once progenitor cells differentiate into oligodendrocytes (Sontheimer et al VSports在线直播. , 1989). Furthermore, the dedifferentiation of retinal glial cells is associated with the upregulation of BK channels (Bringmann et al. , 1999), and their pharmacological inhibition arrests DNA synthesis in these immature cells (Kodal et al. , 2000).
The latter studies in particular suggest an important role for these channels in immature glial cells that are still capable of dividing. Indeed, a number of articles have described the importance of KCa channels in cell proliferation of cells outside the nervous system. For example, keratinocytes (Koegel et al. , 2003), fibroblasts (Pena and Rane, 1999), T-lymphocytes (Khanna et al. , 1999), vascular endothelial cells (Grgic et al. , 2005), breast (Ouadid-Ahidouch et al. , 2004a,b) and prostate (Parihar et al. , 2003) cancer cells all exhibit expresssion of KCa channels, and their inhibition has in all instances been shown to adversely affect cell growth. These findings prompted us to examine glial derived tumor cells with regards to KCa channel expression leading to the identification of prominent BK channels (Liu et al. , 2002; Ransom and Sontheimer, 2001). Importantly, their activity appears to influence cell growth and survival (Weaver et al V体育2025版. , 2004). Little attention, however, has been paid to the expression of IK and SK channels in these cells. To fill this void, the present study was initiated to examine in greater detail the expression and possible function of all known KCa channel types at the RNA, protein and functional level. Much to our surprise, we found transcripts for IK and SK channels yet the only functional channels found were of the BK family. This data agreed with a thorough evaluation of patient biopsies suggesting that glial-derived cancers, unlike most cancers of the body utilize BK channels for their biology.
"VSports" MATERIALS AND METHODS
VSports最新版本 - Cell Culture and Drugs
The glioma cell lines U251MG and D54-MG (World Health Organization Grade IV, glioblastoma multiforme) were a gift from Dr. D VSports. Bigner (Duke University, Durham, N. C. ). STTG-1 cells were obtained from the American Tissue Type Culture Collection (ATCC). We also used primary cultures of glioma cells (GBM 62, GBM50) established from biopsy material from a patients with glioblastoma multiforme, provided by the brain tumor tissue core at the University of Alabama at Birmingham (validated by pathology report). Cells were maintained in Dulbecco’s modified eagle medium (DMEM/F12) (Media Tech, University of Alabama at Birmingham Media Preparation Facility) supplemented with 2 mM glutamine (Media Tech) and 7% heat inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT), at 37°C and 10% CO2. Unless otherwise stated, all reagents were obtained from Sigma. The BK channel antibody G18 was a gift of Dr. Yi Zhou (University of Alabama at Birmingham, Birmingham, AL).
Reverse Transcription PCR
Following the supplied protocol, mRNA was extracted from all cells examined with the reagents provided in the RNAqueous Kit from Ambion (Austin, TX). Any remaining DNA contamination was removed according to the protocol supplied in the DNA-Free kit also from Ambion. The One Step RT-PCR kit (Qiagen, Valencia, CA) contained all the necessary reagents to carryout out both reverse transcription and PCR. Primer sequences targeted against each of the five KCa channels were previously published (Carignani et al., 2002; Liu et al., 2002; Logsdon et al., 1997) and are listed in Table 1. Briefly, all reagents were added to reaction tubes according to manufacturer’s suggestions, and primers were added at a final concentration of 0.6 μM. mRNA was added and the reaction was begun. Reverse transcription was carried out at 50°C for 30 min followed immediately by PCR. The PCR reaction was as follows: 95°C for 10 min, 35 cycles of 94°C for 30 sec, 55°C for 30 sec and 68°C for 1 min followed by a final extension step at 72°C for 10 min. An Eppendorf Mastercycler gradient thermo-cycler was used for both reactions.
TABLE 1. Primer Sequences Used to Assess Glioma Gene Transcription.
| Channel | Gene | Sequence | Size |
|---|---|---|---|
| BK | gBK | 5′-GTT GGG AAG AAC ATT GTT CTT TGT GG-3′ 5′-ATT TAG GTG ACA CTA TAG AAG TGG ACT TTG ACA GAG AAA GTT TG-3′ |
120 bp |
| IK | KCNN4 | 5′-TCA ATC AAG TCC GCT TCC G-3′ 5′-ATT CTG CTG CAG GTC ATA CAG G-3′ |
600 bp |
| SK1 | KCNN1 | 5′-ACC CCT AAA TCT TGG CCA TCG T-3′ 5′-TAG GCG GGT CCT GCT TTA TTC A-3′ |
280 bp |
| SK2 | KCNN2 | 5′-CGA CAA GCA CGT CAC TTA CAA-3′ 5′-CTG ACA TCA GAA CCC GGA TAA-3′ |
210 bp |
| SK3 | KCNN3 | 5′-AAT CTC CGA TAG CCC CAT TG-3′ 5′-TCG CTT CCT GTC ATC TCC TCT T-3′ |
200 bp |
Electrophysiology
Recordings of whole cell currents were made using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA), following standard recording techniques (Hamill et al., 1981). Patch pipettes were made with thin-walled borosilicate glass (World Precision Instruments (TW150F-4), Sarasota, FL) using an upright puller (Narishige Instruments (PP-830), Tokyo, Japan), and typically had resistances of 3-5 MΩ. Current recordings were digitized on-line at 10 kHz and low-pass filtered at 2 kHz, using a Digidata 1200 (Axon Instruments). pClamp 8.2 (Axon Instruments) was used to acquire and store data. Series resistance (Rs) was compensated to 80%, reducing voltage errors, and cells with compensated Rs above 10 MΩ were omitted. U251 and D54 cells cultured on glass coverslips for 1 to 4 days were used in all experiments.
Solutions
Our standard bath solution consisted of the following (in mM): 125 NaCl, 5 KCl, 1.2 MgSO4, 1 CaCl, 1.6 Na2HPO4, 0.4 NaH2PO4, 10.5 glucose, and 32.5 Hepes acid; pH was adjusted to 7.4 using NaOH and osmolarity was ∼300 mOs. IbTX and TEA were added directly to these solutions at concentrations of 100 nM and 2 mM, respectively. Pipette solutions contained the following (in mM): 145 KCl, 1 MgCl2, 10 EGTA, 10 Hepes sodium salt, pH adjusted to 7.3 with Tris-base. As described previously, CaCl2 was added directly to pipette solution the day of use at a concentration of 2 × 10-4 M and 2.8 × 10-3 M resulting in a free Ca2+ concentration of 1.9 × 10-9 M and 7.5 × 10-7 M, respectively (Ransom et al., 2002). Standard bath solution was continuously exchanged at a rate of ∼1 mL min-1.
Western Blotting
A confluent dish of cells was lysed with RIPA buffer (50 mM TrisCl, pH 7.5, 150 mM NaCl, 1% Nondet P-40 (NP-40), 0.5% sodium deoxycholate, 1% SDS) supplemented with protease and phosphatase inhibitor cocktail (Sigma, St. Louis, MO) for 30 min. Protein was collected by centrifugation at 12,000g at 4°C. The supernatant was transferred to a new tube and protein levels were quantified using the DC protein assay kit from Biorad (Hercules, CA). An equal volume of 2× Laemmli-SDS sample buffer containing 600 mM β-mercaptoethanol was added to 10 μg of protein collected from each cell line or tissue sample investigated. All samples were loaded into their own individual well of a 7.5% precast SDS-Page gel (Biorad). Protein separation was performed at a constant 120 V for ∼90 min. Gels were then transferred at 200 mA for 90 min at room temperature onto PVDF paper (Millipore, Bedford, MA). Membranes were blocked in blocking buffer (5% nonfat dried milk, 2% bovine serum albumin, 2% normal goat serum in TBS plus 0.1% Tween 20 (TBST)). The primary antibodies, antiactin, and G18 (BK channel antibody) were used at dilutions of 1:25,000 and 1:4,000, respectively. The antibodies for SK2, SK3, and IK were obtained from Alomone Labs (Jerusalem, Israel), and the SK1 antibody was obtained from Santa Cruz Biotech (Santa Cruz, CA) and diluted according to manufacturers’ instructions. Blots were incubated in primary antibody for 1 h followed by a wash period (3 × 5 min). Membranes were incubated with HRP-conjugated secondary antibodies for 1 h, followed by another wash period (3 × 5 min) and developed using enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL) on Hyperfilm (Amersham).
Immunohistochemistry
Human glioma tissues and corresponding pathology reports were obtained from the Cooperative Human Tissue Network (Midwestern Division). Frozen tissue was cryosectioned into 6-8 μm slices. Staining procedures have been described previously (Lyons et al., 2002). Briefly, frozen sections were thawed at room temperature (RT) for 15 min in a humidified chamber and then reconstituted in PBS for 10 min. Tissues were fixed in 4% paraformaldehyde (PFA) for 20 min and washed twice in PBS. Next, sections were blocked and permeabilized in 10% horse serum and 0.3% Triton in PBS for 1 h. Primary antibody was diluted in PBS and allowed to bind overnight at 4°C followed by 3 × 10 min washes in PBS. Secondary antibody was incubated for 2 h at RT in the dark again followed by three 10-min washes. DAPI was diluted 1:2,000 and applied for 5 min following the second PBS wash. Slices were then mounted and staining was detected with a Zeiss Axiovert 200M inverted microscope (Carl Zeiss, Thornwood NJ) with a 40×-oil Fluar objective (Carl Zeiss).
shRNA Transfection
A pMaxiK+-OFF EGFP was constructed by ligating annealed oligoneucliotides 5′-GATCCCCGCTCTCACCTACTGGGAATGTTTCAAGAGAACATTCCCAGTAGGTGAGAGCTTTTTA-3′ and 3′-GGGCGAGAGTGGATGACCCTTACAAAGTTCTCTTG TAAGGGTCATCCACTCTCGAAAATTCGA-5′ at BglII and HindIII restriction sites of the pZOFF- EGFP plasmid (Terry-Lorenzo et al., 2005). The small hairpin RNA (shRNA) was targeted to the human KCNMA1 cDNA sequence position 1,183-1,203. D54-MG cells were transfected with either pZ-OFF EGFP (control) or pMaxiK+-OFF EGFP using Nucleofector Kit T (Amaxa, Gaithersburg, MD). Briefly, 2 days prior to transfection, D54 cells were passaged to achieve 70-80% confluency on the day of transfection. On the day of transfection, cells were harvested and 2 × 106 cells were mixed with 2 μg of either pMaxiK+-OFF EGFP or pZ-OFF EGFP (as negative control) in 100 μL of the nucleofector solution and then electroporated with the Amaxa Nucleofector (Amaxa, Gaithersburg, MD). Electroporation of cells was completed with Amaxa program T-27 followed by 500 μL of sterile media added. This suspension was transferred to an eppendorf tube and diluted appropriately for plating on coverslips.
Migration Assay
The day before the experiment was performed, 70% confluent dish of U251 cells were rinsed and supplied with serum-free media overnight. Cell culture inserts (BDBiosciences, San Jose, CA) with 8 μm pores were coated overnight with vitronectin (BD Biosciences) at a concentration of 5 μg mL-1 in PBS. The following day, inserts were washed twice with PBS and blocked with 1% fatty acid free bovine serum albumin (faf-BSA) for 1 h. Inserts were then washed twice in PBS and 400 μL migration assay buffer (MAB, 0.1% faf-BSA in serum free media) was added to the bottom of each well. Cells were rinsed once in PBS and were lifted off the dish by the addition of 0.5 mM EGTA for ∼20 min. U251 cells were rinsed twice by centrifugation and resuspended in MAB and counted. 4 × 104 cells were plated on top of each filter and allowed to adhere for 30 min before drug was added. After addition of drug, cells were allowed to migrate for 5 h. Filters were then fixed and stained with crystal violet, the tops were wiped clean of cells, and representative fields were counted with a Zeiss Axiovert 200M microscope with a 10× objective.
Data Analysis
Results were analyzed using Origin (v.6.0, MicroCal Software, Northhampton, MA) and Excel 2000 (Microsoft, Seattle, WA). Significance was determined by oneway ANOVA since all data showed normal distribution. All data reported are mean × S.E.M. and * denotes P < 0.05 unless otherwise stated.
RESULTS
Glioma Cells Express mRNA for All Five of the Ca2+-Activated K+ Channels
Calcium-activated potassium channels of the intermediate and large conductance types have been shown in numerous cancer cell lines to be involved in functions including, but not limited to, cell proliferation (Basrai et al., 2002; Jager et al., 2004; Kuo and Lin-Shiau, 2004; Ouadid-Ahidouch et al., 2004a,b; Parihar et al., 2003), regulatory volume decrease (Khanna et al., 1999; Roman et al., 2002; Weskamp et al., 2000), and cell migration (Bordey et al., 2000; Kraft et al., 2003). Previous evidence from our own laboratory indicates that human gliomas express the large conductance, calcium activated potassium channel (Ransom and Sontheimer, 2001). This channel has been shown to play a role in mitogen induced proliferation (Kodal et al., 2000), potassium induced proliferation (Basrai et al., 2002), migration (Bordey et al., 2000; Kraft et al., 2003), and cell survival following nutrient withdrawal (Weaver et al., 2004). The full complement of calcium activated potassium channels in glioma cells, however, has not been investigated. In an effort to determine which of the five members of this family are expressed in glioma cells, we performed reverse transcription PCR (RT-PCR) on several well established and frequently used glioma cell lines as well as patient biopsies of both nonmalignant and malignant tissues (Fig. 1). The primer sets illustrated in Table 1 have previously been published (Carignani et al., 2002; Liu et al., 2002; Logsdon et al., 1997). Both normal brain and four tumor samples show mRNA transcripts for BK channels. Additionally, the small conductance calcium-activated potassium channel, SK2 was found to be expressed in all of the tumor samples as well as in primary cultured cortical astrocytes. SK1 and SK3 mRNA expression, however, was restricted to tumor biopsies, the D54-MG and U251 cell lines, all three of which are categorized as World Health Organization (WHO) grade IV tumors. By contrast the STTG-1 cell line, classified as a WHO grade III tumor, did not express the mRNA for either of these SK channels. Even more surprising was the expression of transcript for the intermediate conductance (IK) calcium-activated potassium channel in tumor biopsies and glioma cell lines. This channel has been shown in several studies to be absent in brain (Ishii et al., 1997; Logsdon et al., 1997). IK transcripts were not present in tissue from normal brain and primary cultured cortical astrocytes. Similar to mRNA expression for SK1 and SK3, IK mRNA expression seemed to be restricted to the grade IV tumors.
Fig. 1.

RT-PCR demonstrates expression of KCa channel mRNA in both glioma cell lines and biopsies. Primers directed against each of the 5 KCa channels were used and are listed in Table 1. mRNA from normal human brain tissue, human glioma tissue, three glioma cells lines, and primary rat cortical astrocytes were probed for expression of each of the five channels. IK channel transcripts were detected in human tumor tissue and D54 and U251 glioma cell lines. mRNA for BK channels was detected in all samples except for rat cortical astrocytes. SK1 and SK3 had identical expression profiles being found in normal human brain as well as all of the malignant cell samples. They were absent in rat cortical astrocytes. SK2 transcripts were detected in all samples except for normal human brain tissue. Negative controls were conducted in the same manner as all other sample; however, they remained on ice during reverse transcription, to ensure that samples were not contaminated with genomic DNA. Results are representative of three independent experiments.
Glioma Cells Do Not Exhibit Functional IK or SK Currents (VSports)
In light of the finding that glioma cells express mRNA for all five members of the calcium-activated potassium channel family, we set out to determine whether functional currents attributable to each of these channels were detectable by whole-cell patch clamp recordings. The availability of channel specific blockers further allowed a pharmacological isolation. Unlike BK channels, which are gated by both calcium and voltage, SK and IK channels are gated only by calcium. Because of this, all of our experiments were carried out in the presence of 750 nM [Ca2+]i, a concentration that assures activation of such channels. Representative traces from one cell recorded under such conditions are shown in Fig. 2A. The application of the specific SK and IK blockers, apamin (300 nM) and clotrimazole (10 μM), respectively, failed to inhibit any of the whole-cell current in U251 cells (Fig. 2A) or D54-MG cells (data not shown). Recordings from 10 cells each were obtained and normalized data from these cells shows no contribution of either SK or IK channels to the whole-cell currents determined at either +100 mV or -100 mV (Fig. 2B).
Fig. 2.

U251 whole cell currents are not sensitive to either apamin or CLT. (A) Representative traces from whole cell recordings of U251 cells in the presence of 750 nM [Ca2+]i while bathing on normal bath (black line), 300 nM apamin (red line) or 10 μM CLT (blue line). Cells were held at -40 mV and ramped from -120 mV to +120 mV during the course of the experiment. (B) Cumulative data (n = 10 cells each) illustrating the difference in normalized current density before and after application of specific inhibitors of SK (apamin) or IK (CLT) channels. Current densities from all treatments were normalized to their respective control (either normal pipette solution, or 750 nM Ca2+.
In contrast, application of the specific BK channel inhibitor paxilline (2 μM) resulted in near complete inhibition of outwardly rectifying currents as illustrated for a representative cell in Fig. 3A. The top set of traces were recorded with a free Ca2+ concentration of 1.9 nM in the pipette solution, while the bottom traces had an [Ca2+]i of 750 nM. The corresponding current-voltage (IV) plot in Fig. 3B confirms that paxilline completely inhibited the outward current in U251 glioma cells. It also demonstrates that the increase in intracellular calcium resulted in a leftward shift of the activation curve as would be expected from BK channels. Increasing calcium concentration opens BK channels at more negative membrane potentials, thereby resulting in increased whole cell current amplitude at any given voltage (Fig. 3C). Judged by voltage step recordings from 10 cells such as described above, BK channels are responsible for ∼90% of the outward current in all glioma cells, the remaining current being mediated by NPPB sensitive Cl- currents (Ransom et al., 2001).
Fig. 3.

The specific BK channel inhibitor paxilline inhibits ∼90% of the whole cell outward current in glioma cells. (A) Representative traces from two cells before and after application of 2 μM paxilline. Top trace was carried out in the relative absence of intracellular Ca2+, and the bottom traces had 750 nM free Ca2+ in the pipette. (B) Activation curve of BK channels in normal (■) and 750 nM Ca2+ (▲) pipette solution. Application of paxilline completely inhibits activation of BK channels regardless of [Ca2+]i.(C) Current densities before and after treatment with Pax show that BK currents make up almost 90% of the whole cell outward current in glioma cells (n = 10). Current densities under both conditions were normalized to peak current density at 120 mV in normal pipette solution.
Gliomas Only Express BK Channel Protein
The absence of currents attributable to IK or SK channels suggests that glioma cells do not express IK or SK channel protein on the plasma membrane. The possibility remains, however, that the protein for these channels is expressed, but localized intracellularly, and may be recruited to the surface following an appropriate stimulus. Indeed, Olsen et al. have shown that inwardly rectifying potassium channels that are normally expressed on the plasma membrane of mature spinal cord astrocytes are mislocalized in glioma cells (Olsen and Sontheimer, 2004). Alternatively, BK channels have been shown to function intracellularly on the mitochondrial membrane (Xu et al., 2002). To examine these possibilities, we probed whole cell lysates with antibodies specific for each of the five channels for which we detected mRNA. As illustrated in Fig. 4A, IK channels, which have been shown to be present in the K562 leukemia cell line (Lane 8), are not expressed in any glioma cell line (Lane 1,2,3) or biopsy (Lane 4,5). Likewise, protein expression was not found for SK1, SK2, or SK3 channels in glioma cells (Fig. 4B). Western blots, however, did show that BK channel protein expression is found in both glioma cell lines and patient biopsies (Fig. 4B).
Fig. 4.

Western blots demonstrate that only protein for BK Channels is expressed in glioma cell lines. (A) Representative blot from four independent experiments. Specific antibodies to hIK1 were used to probe lysates from three glioma cell lines, two primary human glioma cultures, cortical rat astrocytes, and whole rat brain. The K562 leukemia cell line was used as a positive control for expression of IK channel protein. (B) Representative blot from three independent experiments illustrating a lack of expression of SK1, 2, and 3 in the same lysates as listed above. Whole rat brain lysates were used as a positive control. Specific antibodies directed against BK, SK1, SK2, and SK3 channels were used.
While serving as a convenient model system, glioma cell lines may lack the channel complement expressed in intact patient tissues. To ascertain whether our cell lines recapitulate glioma KCa channel expression in vivo, we obtained biopsies from three patients with glioblastoma multiforme tumors as well as samples from normal (epileptic) patients. Whole cell lysates from all five samples were probed with the respective KCa channel antibodies and the results obtained agree with the data obtained from cell lines (Fig. 5A). In all three glioma cases, only BK channel protein was detected. In addition to Western blots, immunohistochemistry on a fourth patient biopsy demonstrates BK channel expression (Fig. 5B).
Fig. 5.
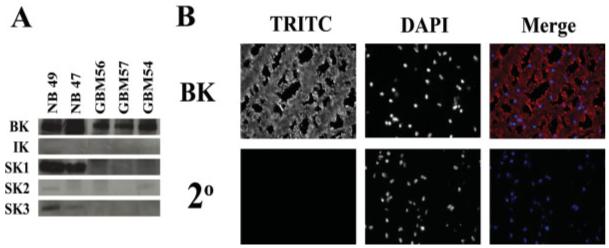
BK channels are expressed in human glioma tissue. (A) Tissue samples from three human GBM biopsies as well as two normal samples were obtained, and tissue lysates were generated and probed for KCa channel expression as with glioma cell lines. Only BK channels were detected in the tumor samples. (B) Tissue slices from a human GBM biopsy were stained with antibodies against BK channels. The images demonstrate expression of BK channels diffusely in human gliomas. The left panel illustrates immunostaining for BK channels, the middle panel for DAPI, and the right panel represents the merge of the two channels.
shRNA Knockdown of BK Eliminates Outward Currents in Glioma Cells
Although the above reagents are quite specific for the channel types of interest, the recent availability of shRNA knockdown approaches now makes it feasible to selectively reduce expression of a single protein in cultured cells. This approach was pursued for both U251 and D54 glioma cells, which were transfected with a plasmid containing a shRNA construct to interfere with the expression of BK channels. This transcript that contains an EGFP tag is expressed under a CMV promoter and hence will be continuously expressed in transfected cells, which can be visualized by EGFP fluorescence. Representative examples of recordings from a transfected cell in which almost complete knockdown was achieved, when compared with controls, are shown in Fig. 6A. shRNA to BK channels reduced whole cell current density by an average of ∼70% (Fig. 6B). As an additional control, a shRNA contruct directed against aquaporin 4 (AQP4), a water channel not expressed in these glioma cell lines, was transfected in to cells to demonstrate the specificity of our construct for BK channels. As expected, the AQP4 shRNA had no effect on whole cell BK currents (Fig. 6B). Furthermore, the decrease in whole cell current corresponded to a decrease in detectable protein (Fig. 6C). This approach did not alter the voltage-dependence or pharmacology of the channels as evidenced by the I/Imax plot in Fig. 6D. Hence, this data further supports the conclusion that glioma cells predominantly express BK type KCa channels.
Fig. 6.

BK shRNA knocks down the majority of the outward voltage dependent current in glioma cells. (A) Representative traces taken from a whole cell recording of a control cell (left) and a cell three days post shRNA transfection (right). Voltage steps in increments of 20 mV were elicited from -40 mV. Following treatment with shRNA, TEA sensitive and the paxilline sensitive (data not shown) component is lost. (B) Bar graph of whole cell current density illustrates a 4-fold reduction in whole-cell conductance following treatment with BK shRNA. ShRNA constructs directed against AQP4 were used as a negative control to illustrate a lack of off-target effects of the shRNA on BK currents. (C) After 3 days of culture following either no treatment (control), BK shRNA treatment, or treatment with vector plasmid, cell lysates were harvested probed for BK channel expression. Actin levels were used as a loading control. BK channel protein is markedly reduced following treatment with BK shRNA. (D) I/Imax plot from voltage step recordings demonstrates that there is no change in voltage dependence of the remaining BK current following treatment.
Inhibition of BK Channels Inhibits Glioma Cell Migration
The ubiquitous expression of BK channels in gliomas of all malignancy grades suggests that these channels play an important role in one or more cellular functions. Previous results from our own laboratory indicate that activation of BK channels via muscarinic receptors inhibits migration of U373 glioma cells (Bordey et al., 2000). Additionally, Kraft et al. have shown that activation of BK channels with the BK channel openers phloretin and NS1619 also inhibit glioma migration in 1321N cells (Kraft et al., 2003). Both studies, however, examined migration in 2D, which do not sufficiently mimic the extracellular environment present in the brain. We have previously suggested that Cl- and K+ efflux support cell shrinkage as cells invade the narrow extracellular space (Sontheimer, 2004). The data thus far suggest that BK channels would be the prime candidate in glioma cells to participate in this K+ efflux, as they are the predominant K+ channel in these cells. To determine whether BK channels may function in such a manner, we utilized a 3D migration system in which glioma cells are placed in a transwell insert with 8 μm pores, a size smaller than the typical diameter of a tumor cell. The small pore size provides a spacial constraint that the cells will have to overcome in order to cross to the other side that has been coated with a chemoattractant, vitronectin, a component of the extracellular matrix. To ensure that any change we saw in the number of cells migrating following treatment was not due to an impediment in adherence, we allowed the cells to attach to the membrane for 30 min before the addition of drugs. The cells were then incubated for 5 h in the presence or absence (control) of 100 nM iberiotoxin, 2 μM paxilline, both BK channel inhibitors, or 30 μM NS1619, a BK channel activator. As we expected, inhibition of BK channels with two specific inhibitors markedly reduced glioma cell migration across the transwell filter (Fig. 7D, n = 3). Indeed, this data further support the notion that glioma cell migration requires the efflux of K+, in addition to the already demonstrated requirement for Cl- efflux (Soroceanu et al., 1999). Interestingly, activation of BK channels with NS1619 failed to enhance glioma cell migration, possibly because BK channels under control conditions are already maximally activated.
Fig. 7.

IbTX and paxilline, two specific inhibitors of BK channels, inhibit glioma cell migration. Representative images from control (A), IbTX treated (B), and paxilline treated (C) cells are shown. Images illustrate cells that have migrated from the top of a transwell filter to the bottom. (D) Cumulative data from three independent experiments. Cells were plated on top of an 8-lm pore transwell filter and allowed to migrate for 4 h in the presence or absence of 100 nM IbTX, 2 μM Pax, or 30 μM NS1619. Cells that had migrated through at the end of 4 h were counted and values were normalized to control. IbTX and Pax inhibited U251 cell migration by 63 and 68%, respectively, while NS1619, a BK channel activator, had no effect on migration.
DISCUSSION
The combination of biochemical, molecular, and pharmacological approaches employed in this study conclusively demonstrate that human malignant glioma cells, in vitro and in vivo, express functional Ca2+-activated K+ channels that belong to the BK channel family derived from the human slo (hslo) gene. Channel inhibition compromises cell invasion as mimicked by commonly used Transwell assays, suggesting that K+ efflux through these channels plays a supportive role in cell invasion. Surprisingly absent was any contribution of Ca2+-activated K+ channels from the IK or SK family of proteins, although their genes were clearly detected by RT-PCR in all samples investigated. SK and particularly IK channels are prominently expressed in many cancer cells, (Barfod et al., 2001; Kraft et al., 2000; Meyer et al., 1999; Ouadid-Ahidouch et al., 2004a,b), and numerous studies have implicated K+ channels, Ca2+-activated K+ channels in particular, in cell cycle progression, migration/invasion, cell volume control, and apoptosis (Attali et al., 1997; Baran, 1996; Begenisich et al., 2004; Bortner et al., 1997; DeCoursey et al., 1996; Ghanshani et al., 2000; Khanna et al., 1999; Maeno et al., 2000; Manikkam et al., 2002; Nehrke et al., 2003; Premack and Gardner, 1991). The expression and function of Ca2+-activated K+ channels often correlates positively with cell proliferation. For example, IK channel expression increases 4-fold upon activation of T-lymphocytes, and inhibition of this channel with the specific inhibitor clotrimazole inhibits T-lymphocyte proliferation (Khanna et al., 1999). Similarly, IK channel blockade in both endothelial cells (Grgic et al., 2005) and fibroblasts (Pena and Rane, 1999) inhibits bFGF induced proliferation. Chronic exposure of keratinocytes to the IK channel activator, 1-EBIO, suppresses IK channel expression and cell proliferation (Koegel et al., 2003). In breast (Ouadid-Ahidouch et al., 2004b), prostate (Parihar et al., 2003), and pancreatic cancers (Jager et al., 2004), treatment of cells with clotrimazole blocks the ability of cells to progress past the G1/S transition in the cell cycle.
How Ca2+-activated K+ channels contribute to cell proliferation is unclear. It is hypothesized that intracellular Ca2+ fluctuates during the cell cycle (Metcalfe et al., 1986), and the activation of growth factor receptors may participate in this process (Reddy, 1994). This in turn would lead to the enhanced activation of these channels that are gated either by calcium alone (IK, SK) or gating is achieved through a combined contribution of increased intracellular Ca2+ and membrane depolarization(BK). In either instance, the resting membrane potential would hyperpolarize, and hence BK channels are the proteins transducing changes in intracellular Ca2+ to changes in transmembrane voltage.
One of the intriguing features of these tumors is their ability to diffusely invade the normal brain. This makes complete surgical resection a challenge. In several past studies, we have emphasized the importance of cell size and volume regulation in cell invasion, particularly noting the importance of Cl- channels in this process (Sontheimer, 2004). The hypothesis is that cells adjust their volume by effluxing Cl- along with K+ and water. Indeed, chlorotoxin, a putative Cl- channel specific inhibitor (DeBin et al., 1993), has been shown to interfere with glioma cells invasion (Soroceanu et al., 1999) and is now in phase II clinical studies for the treatment of gliomas. The pathway(s) through which K+ would be release as cells invade has not been examined thus far. However, findings presented here suggest that BK channels may serve this role in glioma cells. Pharmacological inhibition of BK channels with either 100 nM IbTX or 2 μM paxilline both specific inhibitors of BK channels greatly reduced the ability of glioma cells to transverse a Transwell barrier, a condition that mimics the spatial constraints encountered by cells as they invade the narrow spaces of brain. Using an assay that examines two-dimensional motility rather than invasion, two studies (Bordey et al., 2000; Kraft et al., 2003), including one of our own (Bordey et al., 2000), demonstrated that BK channel activation rather than its inhibition interferes with cell motility/migration. To elucidate whether this difference in results could be due to non specific effects of the drugs used to inhibit BK channels, we extended our pharmacological studies using small hairpin RNAs (shRNA); short strings of oligonucleotides targeted to bind and induce degradation of specific mRNAs, thus preventing protein translation and expression of the protein of interest. We inserted the shRNA sequences into a plasmid vector, which, upon transfection, results in constant transcription of the shRNA constructs in transfected cells and overcomes the need for subsequent transfections to replenish shRNA supply. Using this approach, we were able to demonstrate a 70% knock-down of BK channel expression 3 days following transfection of the plasmid. Unfortunately, the relatively low transfection efficiency (∼35-40%) disallowed use of this system for Transwell migration assays. However, in our electrophysiology experiments in which transfected cells could be individually identified by the expression of EGFP also encoded in the plasmid, we were able to confirm the loss of paxilline-sensitive BK currents. These studies unequivocally identify the currents targeted by paxilline as BK currents. Therefore, the differences with regards to the role of BK channels in invasion versus motility cannot be explained by nonspecific drug actions but rather suggest that BK channels specifically aid in cell invasion, as proposed above.
Interestingly, the activation of BK channels must occur in response to changes intracellular Ca2+ as cells invade. Studies of astrocytes and glial progenitor cells (the nonmalignant counterparts to glioma cells) have clearly demonstrated the importance of calcium signaling in their migration following injury and during development. Specifically, Matyash et al. demonstrated that inhibition of ryanodine receptors (RyRs) with ryanodine significantly slowed astrocyte migration (Matyash et al., 2002). More conclusively, they were able to show that astrocytes cultured from RyR3 knockout mice displayed impaired motility, illustrating the importance of Ca2+ release from intracellular stores in the process of migration. Additionally, Lohr et al. showed that glial cells from the antennal lobe of the sphinx moth exhibit upregulations of voltage gated calcium channels that coincides with the onset of glial cell migration, and blocking these channels with specific inhibitors or chelating free calcium inhibits glial cell migration (Lohr et al., 2005). OPCs have been shown to express P2X and P2Y receptors that when stimulated with specific agonists elicit rises in [Ca2+]i in these cells (Agresti et al., 2005) and stimulates OPC migration. Finally, influx of Ca2+ into glioma cells through Ca2+-permeable AMPA receptors has been shown to be necessary for glioma invasion in vivo (Ishiuchi et al., 2002). Taken together, these studies suggest that various signals and ligands can stimulate intracellular Ca2+ increases during the migration of glia and glioma cells, and these may in turn enhance the activity of Ca2+-actiavted K+ channels to promote K+ efflux as cells invade. Such a mechanism may more widely apply to migratory cells, including nonmalignant glial and neuronal progenitor cells. Moreover, BK channels may be considered as a suitable pharmacological target to inhibit tumor dissemination, and therefore warrants further study.
Acknowledgments
Grant sponsor: NIH; Grant numbers: RO1-NS36692, RO1-NS31234.
REFERENCES
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonte C, Aloisi F, Visentin S. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia. 2005;50:132–144. doi: 10.1002/glia.20160. [DOI] [PubMed] [Google Scholar]
- Attali B, Wang N, Kolot A, Sobko A, Cherepanov V, Soliven B. Characterization of delayed rectifier Kv channels in oligodendrocytes and progenitor cells. J Neurosci. 1997;17:8234–8245. doi: 10.1523/JNEUROSCI.17-21-08234.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia PK, Suzuki R, Benton DC, Jowett AJ, Chen MX, Trezise DJ, Dickenson AH, Moss GW. A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes. J Neurosci. 2005;25:3489–3498. doi: 10.1523/JNEUROSCI.0597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran I. Calcium and cell cycle progression: Possible effects of external perturbations on cell proliferation. Biophys J. 1996;70:1198–1213. doi: 10.1016/S0006-3495(96)79679-0. [DOI (V体育官网入口)] [PMC free article] [PubMed] [Google Scholar]
- Barfod ET, Moore AL, Lidofsky SD. Cloning and functional expression of a liver isoform of the small conductance Ca2+-activated K+ channel SK3. Am J Physiol Cell Physiol. 2001;280:C836–C842. doi: 10.1152/ajpcell.2001.280.4.C836. [DOI] [PubMed] [Google Scholar]
- Barrett JN, Magleby KL, Pallotta BS. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol (Lond) 1982;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basrai D, Kraft R, Bollensdorff C, Liebmann L, Benndorf K, Patt S. BK channel blockers inhibit potassium-induced proliferation of human astrocytoma cells. Neuroreport. 2002;13:403–407. doi: 10.1097/00001756-200203250-00008. [DOI] [PubMed] [Google Scholar]
- Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- Blatz AL, Magleby KL. Calcium-activated potassium channels. Trends Neurosci. 1987;10:463–467. [Google Scholar]
- Bordey A, Sontheimer H, Trouslard J. Muscarinic activation of BK channels induces membrane oscillations in glioma cells and leads to inhibition of cell migration. J Membr Biol. 2000;176:31–40. doi: 10.1007/s00232001073. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Hughes FM, Jr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem. 1997;272:32436–32442. doi: 10.1074/jbc.272.51.32436. ["V体育官网" DOI] [PubMed] [Google Scholar]
- Bringmann A, Francke M, Pannicke T, Biedermann B, Faude F, Enzmann V, Wiedemann P, Reichelt W, Reichenbach A human Muller glial cells: Altered potassium channel activity in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999;40:3316–3323. [PubMed (V体育ios版)] [Google Scholar]
- Carignani C, Roncarati R, Rimini R, Terstappen GC. Pharmacological and molecular characterisation of SK3 channels in the TE671 human medulloblastoma cell line. Brain Res. 2002;939:11–18. doi: 10.1016/s0006-8993(02)02535-0. [DOI (VSports手机版)] [PubMed] [Google Scholar]
- Charles Intercellular calcium waves in glia. Glia. 1998;24:39–49. doi: 10.1002/(sici)1098-1136(199809)24:1<39::aid-glia5>3.0.co;2-w. ["VSports注册入口" DOI] [PubMed] [Google Scholar]
- DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol. 1993;264:C361–C369. doi: 10.1152/ajpcell.1993.264.2.C361. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Kim SY, Silver MR, Quandt FN. Ion channel expression in PMA-differentiated human THP-1 macrophages. J Membr Biol. 1996;152:141–157. doi: 10.1007/s002329900093. [DOI] [PubMed] [Google Scholar]
- Ferrer J, Wasson J, Salkoff L, Permutt MA. Cloning of human pancreatic islet large conductance Ca(2+)-activated K+ channel (hSlo) cDNAs: Evidence for high levels of expression in pancreatic islets and identification of a flanking genetic marker. Diabetologia. 1996;39:891–898. doi: 10.1007/BF00403907. [DOI] [PubMed] [Google Scholar]
- Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, Cahalan MD, Chandy KG. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, Kohler R. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2005;25:704–709. doi: 10.1161/01.ATV.0000156399.12787.5c. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [V体育安卓版 - DOI] [PubMed] [Google Scholar]
- Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M, Sasaki T, Ozawa S. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8:971–978. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- Jager H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol. 2004;65:630–638. doi: 10.1124/mol.65.3.630. [DOI] [PubMed] [Google Scholar]
- Jensen BS, Hertz M, Christophersen P, Madsen LS. The Ca2+-activated K+ channel of intermediate conductance: A possible target for immune suppression. Expert Opin Ther Targets. 2002;6:623–636. doi: 10.1517/14728222.6.6.623. [DOI] [PubMed] [Google Scholar]
- Khanna R, Chang MC, Joiner WJ, Kaczmarek LK, Schlichter LC. Hsk4/hik1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation. J Biol Chem. 1999;274:14838–14849. doi: 10.1074/jbc.274.21.14838. [DOI (VSports)] [PubMed] [Google Scholar]
- Kodal H, Weick M, Moll V, Biedermann B, Reichenbach A, Bringmann Involvement of calcium-activated potassium channels in the regulation of DNA synthesis in cultured Muller glial cells. Invest Ophthalmol Vis Sci. 2000;41:4262–4267. [PubMed] [Google Scholar]
- Koegel H, Alzheimer C. Expression and biological significance of Ca2+-activated ion channels in human keratinocytes. FASEB J. 2001;15:145–154. doi: 10.1096/fj.00-0055com. [DOI] [PubMed] [Google Scholar]
- Koegel H, Kaesler S, Burgstahler R, Werner S, Alzheimer C. Unexpected down-regulation of the hIK1 Ca2+-activated K+ channel by its opener 1-ethyl-2-benzimidazolinone in HaCaT keratinocytes. Inverse effects on cell growth and proliferation. J Biol Chem. 2003;278:3323–3330. doi: 10.1074/jbc.M208914200. [DOI] [PubMed] [Google Scholar]
- Kraft R, Benndorf K, Patt S. Large conductance Ca(2+)-activated K+ channels in human meningioma cells. J Membr Biol. 2000;175:25–33. doi: 10.1007/s002320001052. [DOI] [PubMed] [Google Scholar]
- Kraft R, Krause P, Jung S, Basrai D, Liebmann L, Bolz J, Patt S. BK channel openers inhibit migration of human glioma cells. Pflugers Arch. 2003;446:248–255. doi: 10.1007/s00424-003-1012-4. [DOI] [PubMed] [Google Scholar]
- Kuo TC, Lin-Shiau SY. Decrease in Ca2+-activated K+ conductance in differentiated C6-glioma cells. Neurochem Res. 2004;29:1453–1459. doi: 10.1023/b:nere.0000026411.84915.55. [DOI] [PubMed] [Google Scholar]
- Liu X, Chang Y, Reinhart PH, Sontheimer H, Chang Y. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci. 2002;22:1840–1849. doi: 10.1523/JNEUROSCI.22-05-01840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon NJ, Kang J, Togo JA, Christian EP, Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem. 1997;272:32723–32726. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- Lohr C, Heil JE, Deitmer JW. Blockage of voltage-gated calcium signaling impairs migration of glial cells in vivo. Glia. 2005;50:198–211. doi: 10.1002/glia.20163. ["V体育ios版" DOI] [PubMed] [Google Scholar]
- Lyons SA, O’Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39:162–173. doi: 10.1002/glia.10083. [DOI] [PubMed] [Google Scholar]
- Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci USA. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [VSports手机版 - DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher AD, Kuchel PW. The Gardos channel: A review of the Ca2+-activated K+ channel in human erythrocytes. Int J Biochem Cell Biol. 2003;35:1182–1197. doi: 10.1016/s1357-2725(02)00310-2. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Li Y, Mitchell BM, Mason DE, Freeman LC. Potassium channel antagonists influence porcine granulosa cell proliferation, differentiation, and apoptosis. Biol Reprod. 2002;67:88–98. doi: 10.1095/biolreprod67.1.88. [DOI] [PubMed] [Google Scholar]
- Matyash M, Matyash V, Nolte C, Sorrentino V, Kettenmann H. Requirement of functional ryanodine receptor type 3 for astrocyte migration. FASEB J. 2002;16:84–86. doi: 10.1096/fj.01-0380fje. [DOI] [PubMed] [Google Scholar]
- Metcalfe JC, Moore JP, Smith GA, Hesketh TR. Calcium and cell proliferation. Br Med Bull. 1986;42:405–412. doi: 10.1093/oxfordjournals.bmb.a072159. Review. [DOI] [PubMed] [Google Scholar]
- Meyer R, Schonherr R, Gavrilova-Ruch O, Wohlrab W, Heinemann SH. Identification of ether a go-go and calcium-activated potassium channels in human melanoma cells. J Membr Biol. 1999;171:107–115. doi: 10.1007/s002329900563. ["VSports" DOI] [PubMed] [Google Scholar]
- Nehrke K, Quinn CC, Begenisich T. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol Cell Physiol. 2003;284:C535–C546. doi: 10.1152/ajpcell.00044.2002. ["V体育安卓版" DOI] [PubMed] [Google Scholar]
- Nowak L, Ascher P, Berwald-Netter Y. Ionic channels in mouse astrocytes in culture. J Neurosci. 1987;7:101–109. doi: 10.1523/JNEUROSCI.07-01-00101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. Mislocalization of Kir channels in malignant glia. Glia. 2004;46:63–73. doi: 10.1002/glia.10346. [V体育安卓版 - DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel β-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. ["V体育安卓版" DOI] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Roudbaraki M, Ahidouch A, Delcourt P, Prevarskaya N. Cell-cycle-dependent expression of the large Ca2+-activated K+ channels in breast cancer cells. Biochem Biophys Res Commun. 2004a;316:244–251. doi: 10.1016/j.bbrc.2004.02.041. [VSports app下载 - DOI] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca(2+)-activated K(+) channels in breast cancer cells: Association with cell cycle progression. Am J Physiol Cell Physiol. 2004b;287:C125–C134. doi: 10.1152/ajpcell.00488.2003. ["V体育ios版" DOI] [PubMed] [Google Scholar]
- Parihar AS, Coghlan MJ, Gopalakrishnan M, Shieh CC. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur J Pharmacol. 2003;471:157–164. doi: 10.1016/s0014-2999(03)01825-9. [DOI] [PubMed] [Google Scholar]
- Pena TL, Rane SG. The fibroblast intermediate conductance K(Ca) channel FIK, as a prototype for the cell growth regulatory function of the IK channel family. J Membr Biol. 1999;172:249–257. doi: 10.1007/s002329900601. ["VSports app下载" DOI] [PubMed] [Google Scholar]
- Premack BA, Gardner P. Role of ion channels in lymphocytes. J Clin Immunol. 1991;11:225–238. doi: 10.1007/BF00918180. Review. [DOI] [PubMed] [Google Scholar]
- Quandt FN, MacVicar BA. Calcium activated potassium channels in cultured astrocytes. Neuroscience. 1986;19:29–41. doi: 10.1016/0306-4522(86)90003-5. [DOI] [PubMed] [Google Scholar]
- Ransom CB, Liu X, Sontheimer H. BK channels in human glioma cells have enhanced calcium sensitivity. Glia. 2002;38:281–291. doi: 10.1002/glia.10064. [DOI] [PubMed] [Google Scholar]
- Ransom CB, O’Neal JT, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci. 2001;21:7674–7683. doi: 10.1523/JNEUROSCI.21-19-07674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom CB, Sontheimer H. BK channels in human glioma cells. J Neurophysiol. 2001;85:790–803. doi: 10.1152/jn.2001.85.2.790. [DOI (VSports注册入口)] [PubMed] [Google Scholar]
- Reddy GPV. Cell cycle: Regulatory events in G1 → S transition of mammalian cells. J Cell Biochem. 1994;54:379–386. doi: 10.1002/jcb.240540404. [DOI] [PubMed] [Google Scholar]
- Roman R, Feranchak AP, Troetsch M, Dunkelberg JC, Kilic G, Schlenker T, Schaack J, Fitz JG. Molecular characterization of volume-sensitive SK(Ca) channels in human liver cell lines. Am J Physiol Gastrointest Liver Physiol. 2002;282:G116–G122. doi: 10.1152/ajpgi.2002.282.1.G116. ["V体育官网" DOI] [PubMed] [Google Scholar]
- Sah P, Davies P. Calcium-activated potassium currents in mammalian neurons. Clin Exp Pharmacol Physiol. 2000;27:657–663. doi: 10.1046/j.1440-1681.2000.03317.x. [DOI] [PubMed] [Google Scholar]
- Sah P, Louise Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. Ion channels and amino acid transporters support the growth and invasion of primary brain tumors. Mol Neurobiol. 2004;29:61–71. doi: 10.1385/MN:29:1:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H, Trotter J, Schachner M, Kettenmann H. Channel expression correlates with differentiation stage during development of oligodendrocytes from their precursor cells in culture. Neuron. 1989;2:1135–1145. doi: 10.1016/0896-6273(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Soroceanu L, Manning TJ, Jr, Sontheimer H. Modulation of glioma cell migration and invasion using Cl- and K+ ion channel blockers. J Neurosci. 1999;19:5942–5954. doi: 10.1523/JNEUROSCI.19-14-05942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M. Ca(2+)-activated K+ channels: Molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Swarthout JT, Walling HW. Lysophosphatidic acid: Receptors, signaling and survival. Cell Mol Life Sci. 2000;57:1978–1985. doi: 10.1007/PL00000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-Lorenzo RT, Roadcap DW, Otsuka T, Blanpied TA, Zamorano PL, Garner CC, Shenolikar S, Ehlers MD. Neurabin/protein phosphatase-1 complex regulates dendritic spine morphogenesis and maturation. Mol Biol Cell. 2005;16:2349–2362. doi: 10.1091/mbc.E04-12-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Weaver AK, Liu X, Sontheimer H. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J Neurosci Res. 2004;78:224–234. doi: 10.1002/jnr.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp M, Seidl W, Grissmer S. Characterization of the increase in [Ca(2+)](i) during hypotonic shock and the involvement of Ca(2+)-activated K(+) channels in the regulatory volume decrease in human osteoblast-like cells. J Membr Biol. 2000;178:11–20. doi: 10.1007/s002320010010. ["VSports" DOI] [PubMed] [Google Scholar]
- Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]


