Expression and function of calcium-activated potassium channels in human glioma cells
- PMID: 16817201
- PMCID: PMC2562223 (VSports注册入口)
- DOI: VSports最新版本 - 10.1002/glia.20364
Expression and function of calcium-activated potassium channels in human glioma cells
Abstract
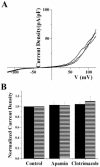
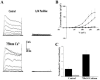
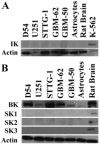
Ca(2+)-activated K(+) (K(Ca)) channels are a unique family of ion channels because they are capable of directly communicating calcium signals to changes in cell membrane potential required for cellular processes including but not limited to cellular proliferation and migration. It is now possible to distinguish three families of K(Ca) channels based on differences in their biophysical and pharmacological properties as well as genomic sequence. Using a combination of biochemical, molecular, and biophysical approaches, we show that human tumor cells of astrocytic origin, i. e. glioma cells, express transcripts for all three family members of K(Ca) channels including BK, IK, and all three SK channel types (SK1, SK2, and SK3). The use of selective pharmacological inhibitors shows prominent expression of currents that are inhibited by the BK channel specific inhibitors iberiotoxin and paxilline. However, despite the presence of transcripts for IK and SK, neither clotrimazole, an inhibitor of IK channels, nor apamin, known to block most SK channels inhibited any current VSports手机版. The exclusive expression of functional BK channels was further substantiated by shRNA knockdown experiments, which selectively reduced iberiotoxin sensitive currents. Western blotting of patient biopsies with antibodies specific for all three KCa channel types further substantiated the exclusive expression of BK type KCa channels in vivo. This finding is in sharp contrast to other cancers that express primarily IK channels. .
V体育官网入口 - Figures




References
-
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonte C, Aloisi F, Visentin S. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia. 2005;50:132–144. - PubMed
-
- Barfod ET, Moore AL, Lidofsky SD. Cloning and functional expression of a liver isoform of the small conductance Ca2+-activated K+ channel SK3. Am J Physiol Cell Physiol. 2001;280:C836–C842. - "V体育2025版" PubMed
Publication types
MeSH terms
- VSports在线直播 - Actions
- "V体育2025版" Actions
- V体育ios版 - Actions
- "VSports在线直播" Actions
- VSports最新版本 - Actions
- "VSports手机版" Actions
Substances (V体育安卓版)
- VSports最新版本 - Actions
- V体育ios版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

