Abstract (VSports手机版)
In Streptococcus mutans, enzyme IIscr and sucrose-6-phosphate hydrolase are two important enzymes in the transport and metabolism of dietary sucrose. The scr regulon of S. mutans is composed of three genes, scrA and scrB, which code for enzyme IIscr and sucrose-6-phosphate hydrolase, respectively, and scrR, which codes for a GalR-LacI-type transcription regulator VSports最新版本. It was previously shown that expression of both scrA and scrB is similarly induced by sucrose. Mutation in the scrR gene resulted in increased expression of scrB relative to that in the wild-type strain. In this study, we employed DNA mobility shift and DNase I protection assays with a purified ScrR-histidine tag fusion protein to examine the DNA binding properties of ScrR to the promoter regions of the scrA and scrB genes. The results showed that ScrR bound specifically to the promoter regions of both scrA and scrB. Two regions with high affinity for ScrR in the promoter sequences of the scrA and scrB genes were identified by DNase I protection assays. One, OC, which includes a 20-bp imperfect inverted-repeat sequence, is located between the two promoters, and the other, OB, is located within the scrB promoter region containing a 37-bp imperfect direct-repeat sequence. Mutations of OB and OC resulted in constitutive transcription and expression of both the scrA and scrB genes. Our results indicated that S. mutans coordinates the activities of enzyme IIscr and sucrose-6-phosphate hydrolase by transcriptional repressor ScrR binding to the promoter regions of the scr regulon.
Streptococcus mutans has been identified as the primary pathogen in the development of human dental caries (10) V体育平台登录. Sugar metabolism, especially that of sucrose, by these bacteria is of great significance in cariogenicity. Although these organisms hydrolyze sucrose extracellularly by secreting fructosyl- and glucosyltransferases that use sucrose for the formation of fructan and colonization-enhancing glucan polymers, respectively (11), the majority of dietary sucrose is metabolized by intracellular enzymes (21). In the metabolism of sucrose, most of the sucrose is normally transported into the cell via the phosphoenolpyruvate:sugar phosphotransferase system (PTS) (13). Enzyme IIscr, located in the cell membrane, internalizes sucrose as sucrose-6-phosphate. This sugar-phosphate is subsequently hydrolyzed by cytoplasmic sucrose-6-phosphate hydrolase (3), yielding glucose-6-phosphate and fructose. After phosphorylation of fructose to fructose-6-phosphate, the phosphorylated products are channeled into the glycolytic pathway. In addition, alternate pathways for sucrose transport, such as the trehalose-PTS (14) and MSM (multiple sugar metabolism) systems (22), have also been identified in these organisms.
Sequence analysis showed that the genes encoding enzyme IIscr and sucrose-6-phosphate hydrolase, scrA and scrB, are tandemly arranged on the S. mutans chromosome but transcribed in opposite directions from individual promoters (Fig. 1) (17). An scrR gene, which was identified downstream from the scrB gene, was shown to be homologous with the galR-lacI repressor family. Previous studies of the scrAB operons in several other gram-positive bacteria have revealed that repressor proteins of the GalR-LacI family negatively control the expression of the sucrose transport proteins VSports注册入口. Inactivation of the sacR gene of Lactococcus lactis resulted in constitutive transcription of the sacBK and sacAR operons with different carbon sources (9). An incomplete inverted-repeat sequence, OB, in the scrB promoter region of the Staphylococcus xylosus chromosome serves as the cis-active sequence mediating sucrose-specific regulation by the ScrR repressor protein (5). In Clostridium beijerinckii, transcription of the scrARBK regulon was shown to be regulated by the scrR gene product (15). Since these bacteria carry homologous putative regulator genes linked to the sucrose operon, it is possible that a similar regulatory mechanism is operative in S. mutans.
"V体育平台登录" FIG. 1.
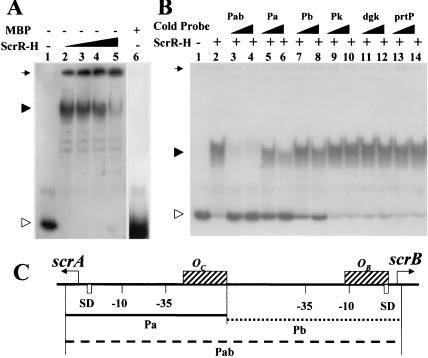
DNA sequences of the promoter regions of the scrA and scrB genes. The −10 and −35 regions of the promoter sequence elements and translation start codons of scrA and scrB were determined previously (15). SD indicates the putative Shine-Dalgarno sequence. Primers for the PCR amplification of the probes used in the DNA mobility shift assay and DNase I protection assay (Fig V体育官网入口. 4) are marked with an arrow over their nucleotide sequences. ScrR binding sites determined by the DNase I protection assay are boxed and named OB and OC. Solid-line arrows under these sequences denote inverted-repeat structures between the two promoters. Dashed-line arrows denoted imperfect direct-repeat structures in the promoter region of the scrB gene.
Previous results with an scrA::lacZ fusion protein in S. mutans GS5 showed that the expression of the scrA gene is elevated in the presence of sucrose relative to that of glucose or fructose (19). More recently, a parallel expression pattern was also observed for the scrB::lacZ fusion in this strain (6). It was suggested that a similar regulatory mechanism controls the expression of these two genes. Inactivation of the scrR gene in S. mutans resulted in increased expression of scrB in the presence of sucrose, glucose, and fructose (6). Accordingly, ScrR might also play a similar role in the regulation of scrA expression in S. mutans VSports在线直播. However, the molecular basis for such regulation was not evident. Since the gene organization of the scrBR and scrA operons in S. mutans is similar to that of the sacAR and sacBK operons of Lactococcus lactis, in which ScrR acts as a repressor of both sacAR transcription and sacBK transcription (9), the involvement of the scrR gene product in the regulation of scrB transcription suggested a potential mechanism for coordinating the regulation of the scr regulon in S. mutans.
The present study, utilizing a purified ScrR-histidine tag fusion protein (ScrR-H) in DNA mobility shift and DNase I protection assays, demonstrates the DNA binding properties of ScrR with potential operators within the promoter regions of the scrA and scrB genes V体育2025版. Mutation of the scrR gene also increased the expression of the scrA::lacZ fusion in S. mutans. Furthermore, mutation of the potential scrR operators OB and OC indicates coregulation of the expression of the scr regulon.
MATERIALS AND METHODS
Bacterial strains.
The S. mutans strains used in this study are listed in Table 1. Escherichia coli DH5α (supE44 hsdR17 recA1 endA1 gyrA96 thi-1 relA1) was utilized as a general cloning host. E. coli M15 (Nals Strs Rifs Thi− Lac− Ara+ Gal+ Mtl− F− RecA+ Uvr+ Lon+) was used for expression of fusion proteins. S. mutans was grown in Todd-Hewitt Broth (Invitrogen, Carlsbad, Calif. ) or defined medium (0. 2% l-glutamic acid, 0. 02% l-cysteine, 0. 09% l-leucine, 0. 1% NH4Cl, 0. 25% K2HPO4, 0. 25% KH2PO4, 0. 4% NaHCO3, 0. 12% MgSO4 · 7H2O, 0. 002% MnCl2 · 4H2O, 0. 002% FeSO4 · 7H2O, 0. 06% Na-pyruvate, 0. 0001% riboflavin, 0. 00005% thiamine-HCl, 0 VSports. 00001% biotin, 0. 0001% nicotinic acid, 0. 00001% p-aminobenzoic acid, 0. 00005% Ca-pantothenate, 0. 0001% pyridoxal-HCl, 0. 00001% folic acid) supplemented with 1% sugar (2). E. coli strains were grown in L broth (Invitrogen), and transformants were selected on L agar plates supplemented with the antibiotics indicated below.
TABLE 1.
Plasmids and S. mutans strains used in this study
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| S. mutans strains | ||
| GS5 | Wild-type strain | 12 |
| SP2 | Spontaneous glucan mutant of GS5 | 12 |
| SP2C4 | scrB::lacZ | 6 |
| SP2C5-2 | scrB::lacZ ΔOB | This study |
| SP2C6-6 | scrB::lacZ ΔOB ΔOC | This study |
| IS3 | Spontaneous glucan mutant of GS5 | 18 |
| IS3AZ2 | scrA::lacZ | 18 |
| AZ24-2 | scrA::lacZ ΔOB | This study |
| AZ23-4 | scrA::lacZ ΔOB ΔOC | This study |
| IS3AZ2 ΔscrR | scrA::lacZ ΔscrR | This study |
| Plasmids | ||
| PQE60 | Vector for cloning gene fused to 3′ terminal | QIAGEN |
| pQEScrR | scrR-H, Ampr | This study |
| pSBL20 | scrB::lacZ Emr | 6 |
| pSBL20B | scrB::lacZ ΔOB Emr | This study |
| pSBL60-I | scrB::lacZ ΔOB ΔOC Emr | This study |
| pAZ4-1 | scrB::spr ΔOB Spr Emr | This study |
| pSCSP-1 | scrB::spr ΔOB ΔOC Spr Emr | This study |
Amp, ampicillin; Em, erythromycin; Sp, spectinomycin.
DNA manipulations.
DNA isolation, restriction endonuclease digestion, PCR, sequencing, Southern blotting, ligation, transformation, and other DNA manipulations were carried out as described by Ausubel et al. (1). Restriction endonucleases and other DNA-modifying enzymes were obtained from Invitrogen, New England Biolabs Inc. (Beverly, Mass.), and Promega Corp. (Madison, Wis.) and used according to the specifications of the suppliers. DNA fragments were isolated from agarose gels by using a QIAEXII kit (QIAGEN, Valencia, Calif.). Double-stranded PCR template sequencing was performed by using a Sequenase 7-deaza-dGTP sequencing kit (U.S. Biochemicals, Cleveland, Ohio).
Overexpression and purification of the fusion protein ScrR-H.
A pair of primers complementary to the scrR gene without a stop codon at the 3′ terminus and an additional NcoI site (underlined) added to the 5′ terminus (dsF-1, 5′-AACCATGGTTGCAAAATTAACAG-3′; ds-3, 5′-CAACACTTTCTCCTGATAAAAGAC-3′) were designed to amplify the scrR gene. After digestion with NcoI and phosphorylation by T4 kinase, the 960-bp DNA fragment was cloned into the NcoI/BglII (blunted with the Klenow fragment)-digested vector pQE-60 (QIAGEN), which contains the six-His tag gene downstream of the multiple cloning site. The recombinant plasmid pQEScrR contained the entire scrR sequence (CATCACCATCACCATCAC) under the control of the T5 promoter and the lac operators. The DNA sequence of scrR in pQEScrR was verified by sequencing.
The transformant harboring plasmid pQEscrR was cultured in 1 liter of L broth containing 100 μg of ampicillin per ml and 25 μg of kanamycin per ml at 37°C to an optical density of 0.5 to 0.6 at 600 nm and then induced by 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After induction at 37°C for 4 h, bacteria were harvested by centrifugation at 4,000 × g for 20 min and resuspended in 20 ml of lysis buffer C (50 mM NaH2PO4, 300 mM NaCl, 2 mM imidazole, pH 8.0). A soluble fraction obtained by sonication and centrifugation was applied to the Ni-nitrilotriacetic acid spin column (QIAGEN), and ScrR-H was eluted according to the directions of the supplier with slight modifications. Protein purity was evaluated by sodium dodecyl sulfate (SDS)-12.5% polyacrylamide gel electrophoresis and Western blotting with RGS · His antibody (QIAGEN).
DNA mobility shift assays.
The DNA mobility shift assays were performed as described by Ausubel et al. (1) with the following modifications. Two to 20 ng of the DNA probe (10,000 to 40,000 cpm) was incubated with the amounts of ScrR-H protein indicated below in 15 μl of binding buffer (12% glycerol, 12 mM HEPES-NaOH [pH 7.9], 4 mM Tris-HCl [pH 7.9], 60 mM KCl, 1 mM EDTA, 1 mM dithiothreitol) at 30°C for 15 min. After incubation, the DNA-protein complexes and free DNA were resolved on nondenaturing 4% polyacrylamide gels with a running buffer containing 6.7 mM Tris · Cl (pH 7.9), 0.3 mM sodium acetate, and 1 mM EDTA. Gels were dried and subjected to autoradiography. The negative controls included a reaction of the DNA probe only without proteins and a reaction of the DNA probe with a nonspecific protein, maltose-binding protein (MBP; New England Biolabs Inc.).
For determination of the ability of ScrR-H to bind the intergenic region between the scrA and scrB genes, three DNA fragments were generated by PCR. Probe Pab from bp 19 to 234 (Fig. 1) contains both promoter regions of the scrA and scrB genes and was amplified by primer Pab-1 (5′-CTACTTTGCTATAATCCATTTGCA-3′) and primer Pab-2 (5′-CATCGTTTATCTACTCCTAATAA-3′). Probe Pa from bp 19 to 139 includes only the scrA promoter with a 20-bp imperfect inverted repeat and was amplified by primers Pab-1 and Pa-2 (5′-TATGTAAAACGATTGACATATTGG-3′). Probe Pb from nucleotides 140 to 234 contains a 37-bp imperfect direct-repeat sequence in the promoter region of scrB and was generated with primers Pb-1 (5′-AAATTCTTTTTTGCAACAAAAG-3′) and Pab-2. Pab was end labeled with [γ-32P]ATP and T4 polynucleotide kinase as described previously (1).
DNase I protection assays.
The DNase I protection assays were carried out using the Core footprinting system (Promega Co.). The reaction mixture (50 μl) containing 10,000 cpm of 32P-labeled DNA probe and the ScrR-H protein (0 to 18 μg) in binding buffer (25 mM Tris · Cl [pH 8.0], 50 mM KCl, 6.25 mM MgCl2, 0.5 mM EDTA, 10% glycerol, 0.5 mM dithiothreitol) was incubated for 10 min on ice according to the directions of the supplier. DNase I was added at a final concentration of 5 U/ml, and the mixture was further incubated at room temperature for 1 min. The digestion was stopped by adding 90 μl of stop solution (200 mM NaCl, 30 mM EDTA, 1% SDS, 100 μg of yeast RNA per ml) and extracted once with phenol-CHCl3 (1:1) and CHCl3. After ethanol precipitation, the pellet was washed with 70% ethanol, dissolved in 10 μl of loading buffer (0.1 M NaOH-formamide [1:2], 0.1% xylene cyanol, 0.1% bromophenol blue), and analyzed on a 6% denatured polyacrylamide gel. For a size ladder, sequencing was performed with the PabE primer (5′-GGAATTCCTACTTTGCTATAATCCATTTGCA-3′) (the nucleotides added for the EcoRI site are underlined) for the sense strand and with the Pdp-3 primer (5′-CAAGGAGACAAAGCTAC-3′) for the antisense strand with a Sequenase kit (U.S. Biochemicals).
DNA templates from bp 19 to 326 (Fig. 1) were generated by PCR with primers PabE and Pdp-3. The DNA fragments were labeled at the 5′ ends with [γ-32P]ATP and T4 polynucleotide kinase. For preparation of the sense strand for DNase I protection assays, the labeled probe was digested with EcoRV and selectively precipitated by ethanol. An antisense strand probe was prepared by digestion with EcoRI after being labeled with 32P.
Construction of the scrR-defective mutants in the S. mutans scrA::lacZ strain.
The chromosomal DNA of SP2ΔscrR (6) was used to transform (12) the S. mutans scrA::lacZ strain IS3AZ2. The resultant mutant, IS3AZ2 ΔscrR, carries a tetracycline resistance gene inserted within the scrR gene.
Mutation of the operators OB and OC in the S. mutans scrA::lacZ strain.
A spontaneous OB deletion mutation plasmid of pSBL20, pAZ4-1 (6) (see below), with a spectinomycin resistance gene (8) instead of the lacZ gene inserted in the scrB gene, was transformed into the S. mutans scrA::lacZ strain IS3AZ2 (19), yielding ΔOB strain AZ24-2. The OB and OC mutation plasmid pSCSP-1 was constructed by replacing the lacZ gene of pSBL60-1 with the same spectinomycin cassette. Transformation of pSCSP-1 into IS3AZ2 yielded the OB and OC mutant AZ23-4.
Mutation of the operators VSports app下载 - OB and OC in the S. mutans scrB::lacZ strain.
A spontaneous OB deletion in plasmid pSBL20 (6) was obtained when recovery of the E. coli strain harboring the plasmid from stock culture yielded pSBL20B. Sequence analysis indicated that a 21-bp fragment from bp 200 to 220 including the Shine-Dalgarno site was deleted (Fig. 1) from pSBL20B. This OB deletion plasmid with a lacZ gene translational insertion in the scrB gene was transformed into the S. mutans SP2 strain, yielding ΔOB strain SP2C5-2. An OC mutation was introduced into pSBL20B by replacing the OC sequence with a 20-bp random sequence (5′-AAGCTTGTGTTACGTACACA-3′) by using an ExSite PCR-based site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). The resultant plasmid, pSBL60-1, contained mutations in both the OB and OC sequences. Transformation of pSBL60-1 into the SP2 strain yielded the OB and OC mutant SP2C6-6.
"V体育官网入口" Northern blots.
The preparation of total RNA from S. mutans was as described previously (6). A DNA fragment of the lacZ gene was generated by PCR with a pair of primers (Lac-1, 5′-CAACGTCGTGACTGGGAAAAC-3′; Lac-2, 5′-CATTACCAGTTGGTCTGGTGTC-3′). The digoxigenin (DIG)-labeled DNA probe was generated with a DIG-High Prime kit (Boehringer Mannheim Corp., Indianapolis, Ind.). The RNA fragments were separated by electrophoresis of the denatured RNA sample (20 μg/lane) in 1% agarose gels containing 3% formaldehyde. The gel was washed three times with 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 10 min to remove formaldehyde, and blotting was carried out with 20× SSC buffer. The blotted membrane was washed with 6× SSC once for 1 min and fixed by UV cross-linking. Hybridization was then carried out with DIG Easy Hyb (Boehringer Mannheim Corp.) with a DIG-labeled probe (0.3 mg/ml) at 50°C according to the directions of the supplier.
Determination of β-galactosidase activity.
The S. mutans ΔOB strain (AZ24-2) and OB-OC mutant (AZ23-4) were grown in defined medium supplemented with 1% concentrations of the sugars indicated below to mid-log phase. Cultures were then centrifuged, and the cells were assayed for β-galactosidase activity as described previously (6).
RESULTS (V体育2025版)
Production and purification of ScrR-H.
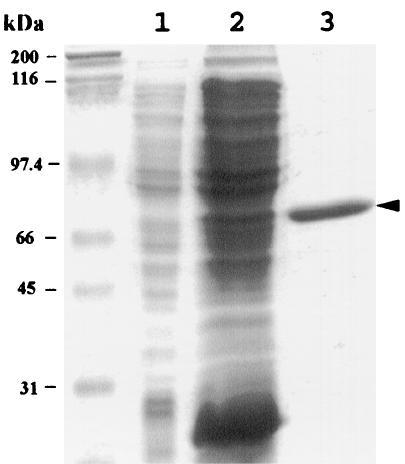
ScrR has homology with the proteins in the GalR-LacI family. The N terminus of this family is important to the specificity of the DNA binding function (23). Therefore, we constructed a protein with a C-terminal fusion. We placed scrR in pQE60 to fuse a six-histidine tag to the COOH-terminal end of the entire scrR sequence. The recombinant plasmid pQEScrR directed the synthesis of ScrR-H. Since the ScrR proteins in several gram-positive bacteria were shown to be autoaggregated, we examined culture and purification conditions to produce the soluble ScrR-H protein. Under the conditions we established, a considerable amount of ScrR-H was produced in the soluble fraction (Fig. 2).
FIG. 2.
Purification of ScrR-H fusion proteins. SDS-polyacrylamide gel electrophoresis results are shown. Molecular mass standards are indicated at the left (from top to bottom: 200, 116, 97.4, 66, 45, and 31 kDa). Lane 1, soluble cell extract before IPTG induction; lane 2, soluble cell extract after IPTG induction; lane 3, purified ScrR-H.
DNA binding properties of ScrR.
In order to assess ScrR binding to the promoter regions of both the scrA and scrB genes, the ScrR fusion protein was utilized since ScrR could not be isolated from either N- or C-terminal-fusion proteins with ScrR due to autoaggregation (unpublished data). Various amounts of purified ScrR-H fusion protein were incubated with the 32P-labeled probe Pab, and shifted bands were detected (Fig. 3). The free probe band could not be detected when ScrR-H was added to the reaction mixture (Fig. 3A, lanes 2 to 4), unlike in the control without ScrR-H (Fig. 3A, lane 1) or with the nonspecific protein MBP (Fig. 3A, lane 6). Instead, the DNA-ScrR complex migrated more slowly and finally was retarded in the sample well as the concentration of ScrR-H increased from 0.3125 to 2.5 μg (Fig. 3A). When unlabeled Pab, Pa, or Pb was added to the reaction mixture, the relative strengths of competition were in the order Pab > Pa > Pb. This finding indicated that there were ScrR binding sites in the Pa region as well as in the Pb region. The affinity of the binding site in Pa was higher than that in Pb (Fig. 3B and C). To further determine binding specificities, excess amounts of unlabeled probes and a series of heterologous DNA fragments, including the scrK promoter region downstream from the scrA gene, which encodes fructokinase (18), the S. mutans dgk structural gene, which encodes diacylglycerol kinase (24), and prtP, a structural gene from Treponema denticola (7), were added to a series of binding reaction mixtures. The results showed that, compared to the other heterologous competitors, the unlabeled Pab DNA fragment at a 10-fold excess competed effectively with 32P-labeled Pab (Fig. 3B).
FIG. 3.
Binding of ScrR-H to the promoter regions of the scrA and scrB genes by DNA mobility shift assays. Lane 1 was a control in which no ScrR-H was present. The positions of ScrR-H-bound (filled arrowhead) and free (open arrowhead) probes are shown. The position of the sample well is also indicated by an arrow. (A) Different amounts of ScrR-H were mixed with 0.02 pmol of 32P-Pab, namely, 0.3125 μg (lane 2), 0.625 μg (lane 3), 1.25 μg (lane 4), and 2.5 μg (lane 5), and 0.02 pmol of 32P-Pab was mixed with 5 μg of MBP (lane 6). (B) Competition experiment in the DNA mobility shift assays in which 32P-Pab was incubated with ScrR-H and different unlabeled DNA fragments. The concentrations of each unlabeled DNA fragment shown above the lanes were 0.4 pmol (lanes 3, 5, 7, 9, 11, and 13) and 0.8 pmol (lanes 4, 6, 8, 10, 12, and 14). Lane 2 was a control in which ScrR-H (15.6 ng) was mixed with 32P-Pab (0.04 pmol). (C) DNA fragments in the promoter regions of the scrA and scrB genes used in DNA mobility shift assays. Probe Pab from bp 19 to 234 contained both promoter regions of the scrA and scrB genes (dashed arrow). Probe Pa from bp 19 to 139 includes only the scrA promoter with a 20-bp imperfect inverted repeat (solid line). Probe Pb from nucleotides 140 to 234 contained a 37-bp imperfect direct-repeat sequence overlapping the Shine-Dalgarno (SD) (open box) region of scrB (dotted line). ScrR binding sites, OB and OC, are marked as hatched boxes. Translation start codons are indicated by bent arrows.
Identification of putative ScrR operator regions in the promoter sequences of scrA and scrB.
Results of the DNA mobility shift assays suggested the possibility of ScrR binding sites in the promoter regions of scrA and scrB. In order to identify these potential operator sequences, DNase I protection assays were carried out by using a 5′-end-labeled 32P-PabE probe amplified with the primer pair PabE and Pdp-3 (Fig. 1). The probe was incubated with serial dilutions of ScrR-H fusion proteins and then subjected to DNase I digestion. The electrophoresis patterns of different amounts of ScrR (Fig. 4, lanes 2 to 4) were compared with a DNase I ladder (Fig. 4, lane 1) and the results of a competition assay in which unlabeled PabE was added to the reaction mixture of ScrR-H and 32P-PabE (Fig. 4, lane 5). The experiment was reproducible for both the sense strand (Fig. 4A) and antisense strand (Fig. 4B) of PabE. The result showed that two regions with high affinity to ScrR were present in the promoter sequences. OB contains a 35-bp imperfect direct repeat (from bp 187 to 221) located in the promoter region of scrB gene (Fig. 1). OC (from bp 120 to 139) containing a 20-bp imperfect inverted-repeat sequence (5′-TATGTCAATCGTTTTACATA-3′) is located between the two promoters (Fig. 1). When 32P-PabE was incubated with 1.2 pmol of ScrR-H, a protected region in the sequence of intergenic region of the scrA and scrB genes, named OC, was observed (Fig. 4A). When the probe was incubated with 6 pmol of ScrR-H, the second protected region in the scrB promoter region, named OB, was observed. These findings are consistent with the results of DNA mobility shift assays, which indicated that the affinity of Pa for ScrR is higher than that of Pb. The result that unlabeled PabE added to the reaction mixture competed with labeled PabE for ScrR binding and protection from DNase I digestion suggested that OB and OC are specific binding sites of ScrR.
FIG. 4.
DNase I protection assay. The sense strand (A) and antisense strand (B) of PabE are shown. (A) In lanes 1 to 4, 32P-PabE (0.12 pmol) was incubated with 0, 0.12, 1.2, and 6 pmol of ScrR-H protein; in lane 5, 10 pmol of unlabeled PabE was added to the reaction mixture used in lane 4. A DNA sequence ladder using the PabE primer as a molecular ruler is shown in lanes 6 to 8. (B) In lanes 1 to 4, 32P-PabE (0.12 pmol) was incubated with 0, 0, 4, 8, and 16 pmol of ScrR-H protein; in lane 5, 10 pmol of unlabeled PabE was added to the reaction mixture used in lane 3. A DNA sequence ladder using the Pdp-3 primer as a molecular ruler is shown in lanes 6 to 8.
Role of ScrR in the regulation of scrA expression. (V体育官网)
In order to determine whether ScrR plays a role as a regulatory protein in affecting scrA expression as it does for scrB (6), an scrR mutation was introduced into an scrA::lacZ strain by double-crossover recombination (see Materials and Methods). The mutated scrR gene in the resultant mutant (IS3AZ2 ΔscrR) was confirmed by Southern blotting with a DIG-labeled scrR probe (data not shown). The wild-type strain and the scrR mutant were grown in the presence of a defined broth containing 1% sucrose. The β-galactosidase activity in the mutant (6.1 ± 0.39 U) was about twofold higher than that in the wild type (3.2 ± 0.23 U) in repeated experiments. Similar results were also observed when these strains were grown with other sugars, such as fructose (3.1 ± 0.4 U in the scrR mutant versus 1.37 ± 0.25 U in the wild-type strain) and glucose (5.88 ± 0.09 U in the scrR mutant versus 3.7 ± 0.5 U in the wild-type strain). These results indicated that ScrR plays a role in the regulation of scrA expression similar to the role it plays with scrB.
"VSports手机版" Increased transcription of the scr regulon by mutation of the potential operators OC and OB.
Based on the sequences of the promoter regions of scrA and scrB (Fig. 1), the ScrR binding sequences appear to be very diverse, which suggested that the functions of these binding sites might be different. OB and OC contain an imperfect direct repeat and an imperfect inverted repeat, respectively, which have been shown to act as operators in GalR-LacI regulation (23). Since ScrR belongs to this group of proteins, we made mutations in the OB and OC regions to demonstrate the function of ScrR-operator interactions in the transcriptional repression of the target genes.
Initially, an OB deletion plasmid (pAZ4-1) and an OB-OC mutation plasmid (pSCSP-1) were constructed and introduced into the S. mutans scrA::lacZ strain IS3AZ2 (10). Mutant AZ24-2 contains a deletion mutation in OB. Besides the OB deletion mutation, mutant AZ23-4 contains an additional OC mutation resulting from the replacement of the OC sequence with a 20-bp random sequence (5′-AAGCTTGTGTTACGTACACA-3′) (Fig. 5A). β-Galactosidase activities were determined in the wild-type strain IS3AZ2, the OB mutant AZ24-2, and the OB-OC mutant AZ23-4. The expression of scrA::lacZ was elevated in both mutants compared to that in the wild-type strain in media containing 1% sucrose as well as in glucose and fructose media (Fig. 5B). The expression of scrA::lacZ was at least twofold higher in the OB mutant (AZ24-2) than in the wild-type strain (IS3AZ2) in all the sugar-containing media. The OB-OC mutant (AZ23-4) showed about four- to fivefold-greater expression of scrA::lacZ than the wild-type strain. These results suggested that the ScrR interaction with OB and OC repressed scrA expression.
FIG. 5.
Mutation of OB and OC in the S. mutans scrB::lacZ strain. (A) Genetic and transcriptional organizations of the sucrose gene cluster of the S. mutans scrA::lacZ strain IS3AZ2, the OB mutant AZ24-2, and the OB-OC double mutant AZ23-4. An OB deletion mutation with a spectinomycin resistance gene, instead of the lacZ gene, was inserted into the scrB gene in the chromosome of ΔOB strain AZ24-2. The OC mutation was constructed by replacing the OC sequence with a 20-bp random sequence in the chromosome of the OB and OC mutant AZ23-4. The arrows indicate repeat sequences. (B) Comparison of the levels of expression of the S. mutans scrA::lacZ fusion in the wild type (IS3AZ2), OB mutant (AZ24-2), and OB plus OC mutant (AZ23-4). Overnight cultures of the S. mutans strains were inoculated into defined media supplemented with 1% glucose, sucrose, or fructose. The cultures were incubated at 37°C until an optical density at 600 nm of 0.8 to 0.9 was reached. β-Galactosidase activities were determined with ο-nitrophenyl-β-galactoside (ONPG) as the substrate. The experiment was carried out in triplicate, and the mean values and standard errors of the β-galactosidase activities are shown.
The OB and OC wild-type plasmid (pSBL20), OB deletion plasmid (pSBL20B), and the OB-OC mutation plasmid (pSBL60-1) were also separately introduced into the S. mutans wild-type strain SP2. SP2C4 is an scrB::lacZ reporter strain with the wild-type promoter region. Mutant SP2C5-2 contains a deletion mutation in OB. Mutant SP2C6-6 contains an additional OC mutation resulting from the replacement of the OC sequence with a 20-bp random sequence (5′-AAGCTTGTGTTACGTACACA-3′), besides the OB deletion mutation (Fig. 6A). Since OB overlaps the ribosome binding site of the scrB gene, deletion of OB in the S. mutans scrB::lacZ fusion strains SP2C5-2 and SP2C6-6 resulted in negligible β-galactosidase activities compared to that of the wild-type scrB::lacZ strain (SP2C4) (data not shown). However, transcription of scrB::lacZ was increased in both mutants relative to that in the wild-type strain in the presence of 1% sucrose in Northern blots in which a DIG-labeled lacZ probe was used (Fig. 6B). The 6.7-kb band corresponds to the polycistronic transcript of the scrB::lacZ and erythromycin resistance genes in the wild-type strain SP2C4. The 5.5-kb band represents the polycistronic transcript of the scrB::lacZ and scrR gene. Transcription in the OB-OC mutant, SP2C6-6, was also enhanced relative to that in the OB mutant, SP2C5-2. The same results were also observed when cells were grown in the presence of glucose and fructose (data not shown). These results were consistent with those for the expression of the scrA::lacZ fusion and suggested that the operators OB and OC not only control the transcription of the scrB gene but also regulate the expression of the scrA gene.
FIG. 6.
Mutation of OB and OC in the S. mutans scrB::lacZ strain. (A) Genetic and transcriptional organizations of the sucrose gene cluster of S. mutans SP2C4 (scrB::lacZ), SP2C5-2 (scrB::lacZ ΔOB), and SP2C6-6 (scrB::lacZ ΔOB ΔOC). The genes (open boxes and open arrows) are shown with the mapped promoters as well as the sizes of the transcripts after they were probed with a DIG-labeled lacZ probe in Northern blots. (B) Northern blot analysis of the RNA samples from S. mutans. Lanes: 1, SP2C4 (scrB::lacZ); 2, SP2C5-2 (scrB::lacZ ΔOB); 3, SP2C6-6(scrB::lacZ ΔOB ΔOC). The cells were grown in defined medium containing 1% sucrose. Sizes of the mRNA are shown.
VSports在线直播 - DISCUSSION
Sucrose metabolism is associated with the pathogenicity of S. mutans in dental plaque. Earlier studies demonstrated that enzyme IIscr and sucrose-6-phosphate hydrolase are two key enzymes in the major PTS pathway transporting sucrose and that sucrose induces the expression of both enzymes (3, 13, 16, 17). Previous results of an scrR deletion mutation downstream of the scrB gene suggested that ScrR acts as a repressor involved in the sucrose induction of the scrB operon. That the expression pattern of scrB in the presence of different sugars is altered by the scrR mutation suggested that the regulation of scrB expression by ScrR is also influenced by sugars other than sucrose. This finding suggested that a complex mechanism is involved in the ScrR regulation of scrB expression, which might also be applicable to scrA expression. Therefore, it was of interest to study the interaction of ScrR with its potential target operator sites. The results of the present DNA mobility shift assays using an ScrR protein with a C-terminal fusion directly demonstrated that ScrR is a DNA binding protein with high affinity for the promoter regions of both the scrA and scrB genes.
It is of interest that a variety of scr gene organizations and regulatory mechanisms are apparent among the microorganisms able to transport and metabolize sucrose (4, 20). For most bacteria, both the scrA and srcB genes are transcribed either within the same operon or independently from the same DNA strand. However, in the mutans streptococci, the scrA and scrB gene homologs are transcribed from opposite DNA strands (16). Recently, divergent sacBK and sacAR operons were characterized for L. lactis strains with similar arrangements on the chromosome such that the sacA gene (encoding sucrose-6-phosphate hydrolase) and the sacB gene (encoding enzyme IIsuc) are transcribed from opposite DNA strands with individual promoters. Following the sacA gene in these organisms is a sacR gene, which codes for a repressor protein with its own promoter. The inactivation of the sacR gene resulted in the constitutive transcription of the sacBK and sacAR operons in the presence of different carbon sources (9). However, it is not clear how the SacR protein interacted with its target operators. For Bacillus subtilis, the regulatory sacT gene involved in antitermination is found upstream of the scrA and scrB genes (4). An incomplete inverted-repeat sequence, OB, in the scrB promoter region of S. xylosus is found to act as a cis factor, which interacts with a repressor, ScrR, to down-regulate the expression of scrB. A similar palindromic sequence was also found in the scrA gene promoter of S. xylosus (5). In S. mutans, the scrR gene is located downstream from the scrB gene and is cotranscribed with this gene (6). It was therefore reasonable to propose that ScrR acts as repressor to regulate the production of sucrose-6-phosphate hydrolase. The two regions with specific affinity for ScrR in the promoter regions of both the scrA and scrB genes suggested potential ScrR operators in this region and possible coregulation of the expression of the scr regulon. Among these ScrR affinity regions, OC is located in the intergenic region between scrA and scrB with a 20-bp imperfect inverted-repeat sequence. It is probably important in the coordination of the regulation of the scrA and scrB genes. Furthermore, OB contains an imperfect direct-repeat sequence within the promoter region of scrB. Interestingly, these regions share little homology with each other.
Inactivation of the scrR gene resulted in increased expression of the scrA::lacZ fusion, suggesting that ScrR acts as a repressor in this operon. In addition, mutation of OB and OC is responsible for the constitutive expression of scrA::lacZ and transcription of scrB::lacZ, suggesting that ScrR repression of the scr regulon is mediated via cis-active elements of the genes. Both genes are regulated via the operators OB and OC. Since scrR is cotranscribed with scrB controlled by the scrB promoter, activation of the scrB promoter will induce ScrR production; subsequent binding of ScrR to the promoter regions of scrA and scrB will reduce the transcription of the scrA and scrB genes. Thus, these might serve as mechanisms to coordinately autoregulate the expression of scrA and scrB.
Moreover, the pattern of expression of the scr regulon in the presence of the three dietary sugars (sucrose > glucose ≫ fructose) was not altered in the operator mutants relative to the pattern in the wild-type strain. This result suggests that ScrR's interaction with OB and OC may not solely be responsible for differential sugar regulation of scrA and scrB expression. Therefore, it is likely that a complex set of interactions involving dietary sugars regulates the expression of the scr regulon in S. mutans. The observation that inactivation of the ScrR-negative regulator led only to a moderate induction in the expression of the scrA and scrBR genes is also compatible with the suggestion that ScrR-independent regulation of this regulon may occur in S. mutans. Additional approaches will be required to examine this hypothesis.
Acknowledgments
This study was supported in part by NIH grant DE03258.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Curent protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 2.Baev, D., R. England, and H. K. Kuramitsu. 1999. Stress-induced membrane association of the Streptococcus mutans GTP-binding protein, an essential G protein, and investigation of its physiological role by utilizing an antisense RNA strategy. Infect. Immun. 67:4510-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chassy, B. M., and E. V. Porter. 1979. Initial characterization of sucrose-6-phosphate hydrolase from Streptococcus mutans and its apparent identity with intracellular invertase. Biochem. Biophys. Res. Commun. 89:307-314. ["V体育官网" DOI] [PubMed] [Google Scholar]
- 4.Debarbouille, M., M. Arnaud, A. Fouet, A. Klier, and G. Rapoport. 1990. The sacT gene regulating the sacPA operon in Bacillus subtilis shares strong homology with transcriptional antiterminators. J. Bacteriol. 172:3966-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gering, M., and R. Bruckner. 1996. Transcriptional regulation of the sucrase gene of Staphylococcus xylosus by the repressor ScrR. J. Bacteriol. 178:462-469. ["VSports在线直播" DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiratsuka, K., B. Wang, Y. Sato, and H. Kuramitsu. 1998. Regulation of sucrose-6-phosphate hydrolase activity in Streptococcus mutans: characterization of the scrR gene. Infect. Immun. 66:3736-3743. ["VSports在线直播" DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishihara, K., T. Miura, H. K. Kuramitsu, and K. Okuda. 1996. Characterization of the Treponema denticola prtP gene encoding a prolyl-phenylalanine-specific protease (dentilisin). Infect. Immun. 64:5178-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBlanc, D. J., L. N. Lee, and J. M. Inamine. 1991. Cloning and nucleotide base sequence analysis of a spectinomycin adenyltransferase AAD(9) determinant from Enterococcus faecalis. Antimicrob. Agents Chemother. 35:1804-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luesink, E. J., J. D. Marugg, O. P. Kuipers, and W. M. de Vos. 1999. Characterization of the divergent sacBK and sacAR operons, involved in sucrose utilization by Lactococcus lactis. J. Bacteriol. 181:1924-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munro, C., S. M. Michalek, and F. L. Macrina. 1991. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect. Immun. 59:2316-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newbrun, E. 1983. Cariology, 2nd ed. Williams and Wilkins, Baltimore, Md.
- 12.Perry, D., L. M. Wondrack, and H. K. Kuramitsu. 1983. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect. Immun. 41:722-727. [DOI (VSports注册入口)] [PMC free article] [PubMed] [Google Scholar]
- 13.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poy, F., and G. R. Jacobson. 1990. Evidence that a low-affinity sucrose phosphotransferase activity in Streptococcus mutans GS-5 is a high-affinity trehalose uptake system. Infect. Immun. 58:1479-1480. ["VSports app下载" DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid, S. J. M. S. Rafudeen, and N. G. Leat. 1999. The genes controlling sucrose utilization in Clostridium beijerinckii NCIMB 8052 constitute an operon. Microbiology 145:1461-1472. [DOI] [PubMed] [Google Scholar]
- 16.Sato, Y., and H. K. Kuramitsu. 1988. Sequence analysis of the Streptococcus mutans scrB gene. Infect. Immun. 56:1956-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato, Y., F. Poy, G. R. Jacobson, and H. K. Kuramitsu. 1989. Characterization and sequence analysis of the scrA gene encoding enzyme IIScr of the Streptococcus mutans phosphoenolpyruvate-dependent sucrose phosphotransferase system. J. Bacteriol. 171:263-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato, Y., Y. Yamamoto, H. Kizaki, and H. K. Kuramitsu. 1993. Isolation, characterization and sequence analysis of the scrK gene encoding fructokinase of Streptococcus mutans. J. Gen. Microbiol. 139:921-927. [DOI] [PubMed] [Google Scholar]
- 19.Sato, Y., Y. Yamamoto, R. Suzuki, H. Kizaki, and H. K. Kuramitsu. 1991. Construction of scrA::lacZ gene fusions to investigate regulation of the sucrose PTS of Streptococcus mutans. FEMS Microbiol. Lett. 79:339-346. [VSports app下载 - DOI] [PubMed] [Google Scholar]
- 20.Steinmetz, M. 1993. Carbohydrate catabolism: pathways, enzymes, genetic regulation and evolution. American Society for Microbiology, Washington, D.C.
- 21.Tanzer, J. M., B. M. Chassy, and M. I. Krichevsky. 1971. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim. Biophys. Acta 261:379-387. ["V体育ios版" DOI] [PubMed] [Google Scholar]
- 22.Tao, L., I. C. Sutcliffe, R. R. Russell, and J. J. Ferretti. 1993. Transport of sugars, including sucrose, by the msm transport system of Streptococcus mutans. J. Dent. Res. 72:1386-1390. [DOI] [PubMed] [Google Scholar]
- 23.Weickert, M. J., and S. Adhya. 1992. A family of bacterial regulators homologous to Gal and Lac repressors. J. Biol. Chem. 267:15869-15874. [V体育官网入口 - PubMed] [Google Scholar]
- 24.Yamashita, Y., T. Takehara, and H. K. Kuramitsu. 1993. Molecular characterization of a Streptococcus mutans mutant altered in environmental stress responses. J. Bacteriol. 175:6220-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]