Stress-induced membrane association of the Streptococcus mutans GTP-binding protein, an essential G protein, and investigation of its physiological role by utilizing an antisense RNA strategy
- PMID: 10456893
- PMCID: PMC96771
- DOI: 10.1128/IAI.67.9.4510-4516.1999
Stress-induced membrane association of the Streptococcus mutans GTP-binding protein, an essential G protein, and investigation of its physiological role by utilizing an antisense RNA strategy
"VSports注册入口" Abstract
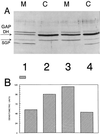
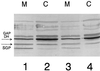
SGP (for Streptococcus GTP-binding protein) is a Streptococcus mutans essential GTPase which has significant sequence identity to the previously identified Escherichia coli Era protein and to numerous other prokaryotic GTPase proteins of unknown function. Recent studies in our laboratory have addressed the possible role of SGP in the stress response of the oral pathogen S. mutans. Here we report that during growth in the early stationary phase, and in response to elevated temperatures or acidic pH, the distribution of SGP between the cytoplasm and the membranes of S. mutans cells varies. Immunoblot analysis of soluble and membrane protein fractions collected from the mid-log and early stationary growth phases of bacterial populations grown at normal temperature (37 degrees C) and at the elevated temperature of 43 degrees C, or at acidic pH, demonstrated that the total amount of SGP increased with the age of the bacterial culture, elevated temperature, or acidic pH. Furthermore, it was established that a substantial amount of SGP is associated with the membrane fraction under stress conditions. In order to investigate the physiological role of SGP, we constructed an S. mutans strain capable of chromosomal sgp antisense RNA expression, which interferes with the normal information processing of the sgp gene. Utilizing this strain, we determined conditions whereby the streptococcal cells can be depleted of SGP, thus avoiding the problem of constructing a conditional lethal system. From the results of measurements of the nucleotide pools extracted from the antisense strain and its isogenic counterpart, we propose that one of the physiological roles of SGP is regulation and modulation of the GTP/GDP ratio under different growth conditions. Moreover, we observed that in SGP-depleted cells the levels of glucan-binding protein A (GbpA) substantially increased, suggesting that GbpA may have stress response-related physiological functions VSports手机版. Finally, the potential applications of the antisense RNA approach that we employed are discussed. .
Figures




V体育平台登录 - References
-
- Banas J A, Potvin H C, Singh R N. The regulation of Streptococcus mutans glucan-binding protein A expression. FEMS Microbiol Lett. 1997;154:289–292. - PubMed
-
- Bourne H R, Sanders D A, McCormick F. The GTPase super-family: conserved structure and molecular mechanism. Nature (London) 1991;349:117–127. - PubMed
-
- Boyd D A, Cvitkovitch D G, Hamilton I R. Sequence, expression, and function of the gene for the nonphosphorylating, NADP-dependent glyceraldehyde-3-phosphate dehydrogenase of Streptococcus mutans. J Bacteriol. 1995;177:2622–2627. - VSports注册入口 - PMC - PubMed
-
- Cashel M, Gentry D R, Hernandez J, Vinella D. The stringent response. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 1458–1496.
Publication types
- "V体育官网" Actions
MeSH terms
- V体育2025版 - Actions
- "VSports app下载" Actions
- "VSports app下载" Actions
- "VSports注册入口" Actions
- "VSports在线直播" Actions
- VSports在线直播 - Actions
- "V体育官网" Actions
Substances
- "VSports注册入口" Actions
- V体育2025版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

