Control of enzyme IIscr and sucrose-6-phosphate hydrolase activities in Streptococcus mutans by transcriptional repressor ScrR binding to the cis-active determinants of the scr regulon
- PMID: 13129950
- PMCID: PMC193960
- DOI: 10.1128/JB.185.19.5791-5799.2003
Control of enzyme IIscr and sucrose-6-phosphate hydrolase activities in Streptococcus mutans by transcriptional repressor ScrR binding to the cis-active determinants of the scr regulon (V体育安卓版)
Abstract (VSports)
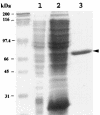
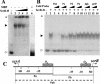
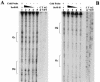
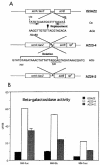
In Streptococcus mutans, enzyme II(scr) and sucrose-6-phosphate hydrolase are two important enzymes in the transport and metabolism of dietary sucrose. The scr regulon of S. mutans is composed of three genes, scrA and scrB, which code for enzyme II(scr) and sucrose-6-phosphate hydrolase, respectively, and scrR, which codes for a GalR-LacI-type transcription regulator. It was previously shown that expression of both scrA and scrB is similarly induced by sucrose. Mutation in the scrR gene resulted in increased expression of scrB relative to that in the wild-type strain. In this study, we employed DNA mobility shift and DNase I protection assays with a purified ScrR-histidine tag fusion protein to examine the DNA binding properties of ScrR to the promoter regions of the scrA and scrB genes. The results showed that ScrR bound specifically to the promoter regions of both scrA and scrB. Two regions with high affinity for ScrR in the promoter sequences of the scrA and scrB genes were identified by DNase I protection assays. One, O(C), which includes a 20-bp imperfect inverted-repeat sequence, is located between the two promoters, and the other, O(B), is located within the scrB promoter region containing a 37-bp imperfect direct-repeat sequence. Mutations of O(B) and O(C) resulted in constitutive transcription and expression of both the scrA and scrB genes VSports手机版. Our results indicated that S. mutans coordinates the activities of enzyme II(scr) and sucrose-6-phosphate hydrolase by transcriptional repressor ScrR binding to the promoter regions of the scr regulon. .
Figures

VSports最新版本 - References
-
- Ausubel, F. M., R. Brent, R. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Curent protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
-
- Chassy, B. M., and E. V. Porter. 1979. Initial characterization of sucrose-6-phosphate hydrolase from Streptococcus mutans and its apparent identity with intracellular invertase. Biochem. Biophys. Res. Commun. 89:307-314. - PubMed
Publication types (VSports手机版)
- Actions (VSports在线直播)
MeSH terms
- Actions (V体育官网入口)
- V体育安卓版 - Actions
- V体育ios版 - Actions
- VSports app下载 - Actions
- "VSports最新版本" Actions
- "VSports在线直播" Actions
- "VSports" Actions
- Actions (V体育安卓版)
- Actions (V体育官网入口)
"V体育平台登录" Substances
- "VSports" Actions
- "VSports" Actions
- "V体育安卓版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous (VSports)

