"VSports注册入口" Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induce Post-Translational Modifications of AKAP121, DRP1, and OPA1 That Promote Mitochondrial Fission
- PMID: 29092894
- PMCID: "VSports app下载" PMC5756120
- DOI: 10.1161/CIRCRESAHA.117.311307
V体育安卓版 - Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induce Post-Translational Modifications of AKAP121, DRP1, and OPA1 That Promote Mitochondrial Fission
Abstract
Rationale: Cardiac lipotoxicity, characterized by increased uptake, oxidation, and accumulation of lipid intermediates, contributes to cardiac dysfunction in obesity and diabetes mellitus. However, mechanisms linking lipid overload and mitochondrial dysfunction are incompletely understood VSports手机版. .
Objective: To elucidate the mechanisms for mitochondrial adaptations to lipid overload in postnatal hearts in vivo. V体育安卓版.
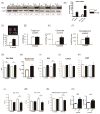
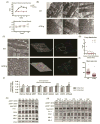
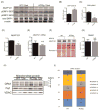
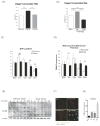
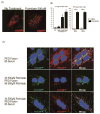
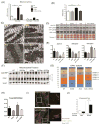
Methods and results: Using a transgenic mouse model of cardiac lipotoxicity overexpressing ACSL1 (long-chain acyl-CoA synthetase 1) in cardiomyocytes, we show that modestly increased myocardial fatty acid uptake leads to mitochondrial structural remodeling with significant reduction in minimum diameter. This is associated with increased palmitoyl-carnitine oxidation and increased reactive oxygen species (ROS) generation in isolated mitochondria. Mitochondrial morphological changes and elevated ROS generation are also observed in palmitate-treated neonatal rat ventricular cardiomyocytes. Palmitate exposure to neonatal rat ventricular cardiomyocytes initially activates mitochondrial respiration, coupled with increased mitochondrial polarization and ATP synthesis. However, long-term exposure to palmitate (>8 hours) enhances ROS generation, which is accompanied by loss of the mitochondrial reticulum and a pattern suggesting increased mitochondrial fission. Mechanistically, lipid-induced changes in mitochondrial redox status increased mitochondrial fission by increased ubiquitination of AKAP121 (A-kinase anchor protein 121) leading to reduced phosphorylation of DRP1 (dynamin-related protein 1) at Ser637 and altered proteolytic processing of OPA1 (optic atrophy 1) V体育ios版. Scavenging mitochondrial ROS restored mitochondrial morphology in vivo and in vitro. .
Conclusions: Our results reveal a molecular mechanism by which lipid overload-induced mitochondrial ROS generation causes mitochondrial dysfunction by inducing post-translational modifications of mitochondrial proteins that regulate mitochondrial dynamics. These findings provide a novel mechanism for mitochondrial dysfunction in lipotoxic cardiomyopathy VSports最新版本. .
Keywords: heart failure; metabolism; mitochondrial dynamics; oxidative stress; reactive oxygen species. V体育平台登录.
© 2017 American Heart Association, Inc.
Conflict of interest statement
Figures (VSports)


Comment in
-
What You Eat Affects Your Shape.Circ Res. 2018 Jan 5;122(1):8-10. doi: 10.1161/CIRCRESAHA.117.312335. Circ Res. 2018. PMID: 29301836 Free PMC article. No abstract available.
V体育2025版 - References
-
- Lopaschuk GD, Collins-Nakai RL, Itoi T. Developmental changes in energy substrate use by the heart. Cardiovasc Res. 1992;26:1172–80. - "V体育平台登录" PubMed
-
- Lopaschuk GD, Witters LA, Itoi T, Barr R, Barr A. Acetyl-CoA carboxylase involvement in the rapid maturation of fatty acid oxidation in the newborn rabbit heart. J Biol Chem. 1994;269:25871–8. - PubMed
Publication types (VSports注册入口)
- VSports注册入口 - Actions
MeSH terms
- V体育官网 - Actions
- Actions (VSports在线直播)
- Actions (VSports在线直播)
- V体育官网入口 - Actions
- Actions (V体育平台登录)
- VSports手机版 - Actions
- Actions (V体育官网)
- V体育官网入口 - Actions
VSports注册入口 - Substances
- "V体育2025版" Actions
Grants and funding
- T32 HL007344/HL/NHLBI NIH HHS/United States
- R01 DK064989/DK/NIDDK NIH HHS/United States (VSports app下载)
- P30 DK079626/DK/NIDDK NIH HHS/United States
- "VSports手机版" R37 HL042873/HL/NHLBI NIH HHS/United States
- R01 HL108379/HL/NHLBI NIH HHS/United States (VSports在线直播)
- U54 DK110858/DK/NIDDK NIH HHS/United States
- "V体育2025版" U01 HL087947/HL/NHLBI NIH HHS/United States
- S10 RR024761/RR/NCRR NIH HHS/United States
- FS/15/3/31047/BHF_/British Heart Foundation/United Kingdom
- VSports最新版本 - R01 HL127764/HL/NHLBI NIH HHS/United States
- P20 HL113444/HL/NHLBI NIH HHS/United States
- S10 RR025439/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育2025版 - Research Materials
"V体育官网入口" Miscellaneous

