Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells
- PMID: 23709681
- PMCID: PMC3746979
- DOI: 10.4049/jimmunol.1300657
V体育平台登录 - Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells
Abstract
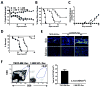
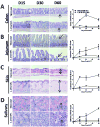
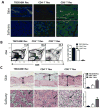
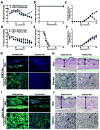
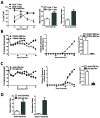
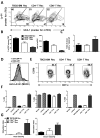
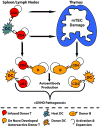
Prevention of chronic graft-versus-host disease (cGVHD) remains a major challenge in allogeneic hematopoietic cell transplantation (HCT) owing to limited understanding of cGVHD pathogenesis and lack of appropriate animal models VSports手机版. In this study, we report that, in classical acute GVHD models with C57BL/6 donors and MHC-mismatched BALB/c recipients and with C3H. SW donors and MHC-matched C57BL/6 recipients, GVHD recipients surviving for >60 d after HCT developed cGVHD characterized by cutaneous fibrosis, tissue damage in the salivary gland, and the presence of serum autoantibodies. Donor CD8(+) T cells were more potent than CD4(+) T cells for inducing cGVHD. The recipient thymus and de novo-generated, donor-derived CD4(+) T cells were required for induction of cGVHD by donor CD8(+) T cells but not by donor CD4(+) T cells. Donor CD8(+) T cells preferentially damaged recipient medullary thymic epithelial cells and impaired negative selection, resulting in production of autoreactive CD4(+) T cells that perpetuated damage to the thymus and augmented the development of cGVHD. Short-term anti-CD4 mAb treatment early after HCT enabled recovery from thymic damage and prevented cGVHD. These results demonstrate that donor CD8(+) T cells cause cGVHD solely through thymic-dependent mechanisms, whereas CD4(+) T cells can cause cGVHD through either thymic-dependent or independent mechanisms. .
Conflict of interest statement (VSports在线直播)
All authors declare no conflict of interest
Figures

VSports注册入口 - References
-
- Martin PJ, Rowley SD, Anasetti C, Chauncey TR, Gooley T, Petersdorf EW, van Burik JA, Flowers ME, Storb R, Appelbaum FR, Hansen JA. A phase I-II clinical trial to evaluate removal of CD4 cells and partial depletion of CD8 cells from donor marrow for HLA-mismatched unrelated recipients. Blood. 1999;94:2192–2199. - PubMed (VSports手机版)
-
- Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. - PubMed
-
- Shlomchik WD, Lee SJ, Couriel D, Pavletic SZ. Transplantation’s greatest challenges: advances in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:2–10. - PubMed
Publication types
- "V体育官网" Actions
- "V体育官网" Actions
V体育2025版 - MeSH terms
- "VSports" Actions
- "VSports" Actions
- "V体育官网入口" Actions
- V体育官网入口 - Actions
- Actions (V体育官网入口)
- Actions (V体育ios版)
- VSports在线直播 - Actions
- Actions (VSports)
- VSports - Actions
- Actions (V体育官网入口)
- V体育ios版 - Actions
- "VSports app下载" Actions
Grants and funding (VSports注册入口)
LinkOut - more resources
VSports手机版 - Full Text Sources
Other Literature Sources
Research Materials (VSports)

