"VSports在线直播" TMEM16F (Anoctamin 6), an anion channel of delayed Ca(2+) activation
- PMID: 23630341
- PMCID: PMC3639583
- DOI: V体育安卓版 - 10.1085/jgp.201210861
TMEM16F (Anoctamin 6), an anion channel of delayed Ca(2+) activation
Abstract
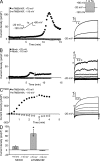
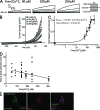
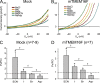
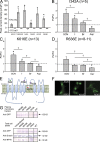
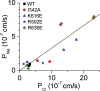
Members of the TMEM16 (Anoctamin) family of membrane proteins have been shown to be essential constituents of the Ca(2+)-activated Cl(-) channel (CaCC) in many cell types. In this study, we have investigated the electrophysiological properties of mouse TMEM16F. Heterologous expression of TMEM16F in HEK293 cells resulted in plasma membrane localization and an outwardly rectifying ICl,Ca that was activated with a delay of several minutes. Furthermore, a significant Na(+) current was activated, and the two permeabilities were correlated according to PNa = 0. 3 PCl. The current showed an EC50 of 100 µM intracellular free Ca(2+) concentration and an Eisenman type 1 anion selectivity sequence of PSCN > PI > PBr > PCl > PAsp. The mTMEM16F-associated ICl,Ca was abolished in one mutant of the putative pore region (R592E) but retained in two other mutants (K616E and R636E). The mutant K616E had a lower relative permeability to iodide, and the mutant R636E had an altered anion selectivity sequence (PSCN = PI = PBr = PCl > PAsp). Our data provide evidence that TMEM16F constitutes a Ca(2+)-activated anion channel or a pore-forming subunit of an anion channel with properties distinct from TMEM16A. VSports手机版.
"VSports在线直播" Figures



VSports最新版本 - References
Publication types
"VSports手机版" MeSH terms
- "VSports在线直播" Actions
- Actions (VSports最新版本)
- "V体育官网" Actions
- "V体育官网入口" Actions
- "V体育平台登录" Actions
Substances
- V体育官网 - Actions
- "V体育安卓版" Actions
- "V体育官网入口" Actions
VSports - LinkOut - more resources
Full Text Sources (V体育ios版)
Other Literature Sources
Research Materials
Miscellaneous

