Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer (V体育2025版)
- PMID: 31534546
- PMCID: PMC6735518
- DOI: 10.7150/thno.36283
"VSports手机版" Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer
"VSports在线直播" Abstract
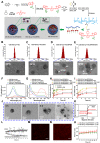
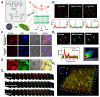
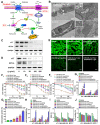
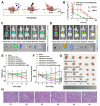
Rationale: Ferroptosis is a regulated process of cell death caused by iron-dependent accumulation of lipid hydroperoxides (LPO). It is sensitive to epithelial-to-mesenchymal transition (EMT) cells, a well-known therapy-resistant state of cancer. Previous studies on nanomaterials did not investigate the immense value of ferroptosis therapy (FT) in epithelial cell carcinoma during EMT. Herein, we describe an EMT-specific nanodevice for a comprehensive FT strategy involving LPO burst. Methods: Mitochondrial membrane anchored oxidation/reduction response and Fenton-Reaction-Accelerable magnetic nanophotosensitizer complex self-assemblies loading sorafenib (CSO-SS-Cy7-Hex/SPION/Srfn) were constructed in this study for LPO produced to overcome the therapy-resistant state of cancer. Both in vitro and in vivo experiments were performed using breast cancer cells to investigate the anti-tumor efficacy of the complex self-assemblies. Results: The nano-device enriched the tumor sites by magnetic targeting of enhanced permeability and retention effects (EPR), which were disassembled by the redox response under high levels of ROS and GSH in FT cells. Superparamagnetic iron oxide nanoparticles (SPION) released Fe2+ and Fe3+ in the acidic environment of lysosomes, and the NIR photosensitizer Cy7-Hex anchored to the mitochondrial membrane, combined sorafenib (Srfn) leading to LPO burst, which was accumulated ~18-fold of treatment group in breast cancer cells. In vivo pharmacodynamic test results showed that this nanodevice with small particle size and high cytotoxicity increased Srfn circulation and shortened the period of epithelial cancer treatment VSports手机版. Conclusion: Ferroptosis therapy had a successful effect on EMT cells. These findings have great potential in the treatment of therapy-resistant epithelial cell carcinomas. .
Keywords: Ferroptosis; epithelial-to-mesenchymal transition; lipid hydroperoxides V体育安卓版. .
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References (V体育平台登录)
-
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. - PubMed
-
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–811. - PubMed (V体育官网入口)
-
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. - PubMed
-
- Nicholas Y, Moritz VR, Elma Z, Bauer AJ, Seok YW, Fridman DJ. et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–8. - VSports在线直播 - PMC - PubMed
Publication types
MeSH terms
- "VSports在线直播" Actions
- Actions (V体育ios版)
- Actions (V体育安卓版)
- "V体育官网" Actions
- "VSports在线直播" Actions
- Actions (VSports)
- VSports - Actions
Substances (V体育2025版)
- VSports最新版本 - Actions
- "V体育安卓版" Actions
"V体育安卓版" LinkOut - more resources
Full Text Sources
Miscellaneous

