CCR7 defines a precursor for murine iNKT cells in thymus and periphery
- PMID: 30102153
- PMCID: PMC6115192 (VSports)
- DOI: V体育2025版 - 10.7554/eLife.34793
CCR7 defines a precursor for murine iNKT cells in thymus and periphery
VSports手机版 - Abstract
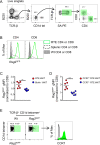
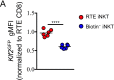
The precise steps of iNKT subset differentiation in the thymus and periphery have been controversial. We demonstrate here that the small proportion of thymic iNKT and mucosal associated invariant T cells that express CCR7 represent a multi-potent progenitor pool that gives rise to effector subsets within the thymus. Using intra-thymic labeling, we also showed that CCR7+ iNKT cells emigrate from the thymus in a Klf2 dependent manner, and undergo further maturation after reaching the periphery. Ccr7 deficiency impaired differentiation of iNKT effector subsets and localization to the medulla. Parabiosis and intra-thymic transfer showed that thymic NKT1 and NKT17 were resident-they were not derived from and did not contribute to the peripheral pool. Finally, each thymic iNKT effector subset produces distinct factors that influence T cell development. Our findings demonstrate how the thymus is both a source of iNKT progenitors and a unique site of tissue dependent effector cell differentiation. VSports手机版.
Keywords: T cell development; iNKT cells; immunology; inflammation; mouse; thymus V体育安卓版. .
© 2018, Wang et al.
Conflict of interest statement
HW, KH No competing interests declared
Figures (V体育安卓版)











References
-
- Allende ML, Zhou D, Kalkofen DN, Benhamed S, Tuymetova G, Borowski C, Bendelac A, Proia RL. S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2008;22:307–315. doi: 10.1096/fj.07-9087com. - DOI - PubMed
-
- Berzins SP, McNab FW, Jones CM, Smyth MJ, Godfrey DI. Long-Term retention of mature NK1.1+ NKT Cells in the Thymus. The Journal of Immunology. 2006;176:4059–4065. doi: 10.4049/jimmunol.176.7.4059. - "VSports手机版" DOI - PubMed
"V体育平台登录" Publication types
- Actions (VSports在线直播)
MeSH terms (V体育官网)
- "V体育安卓版" Actions
- VSports手机版 - Actions
- V体育ios版 - Actions
- "VSports在线直播" Actions
Substances
- V体育安卓版 - Actions
- V体育平台登录 - Actions
