"V体育安卓版" Tryparedoxin peroxidase-deficiency commits trypanosomes to ferroptosis-type cell death
- PMID: 30047863
- PMCID: PMC6117152
- DOI: "V体育官网入口" 10.7554/eLife.37503
Tryparedoxin peroxidase-deficiency commits trypanosomes to ferroptosis-type cell death
Abstract
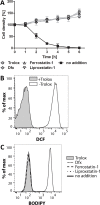
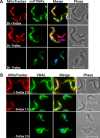
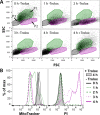
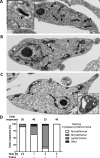
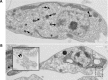
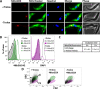
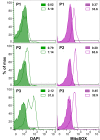
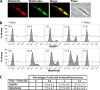
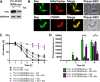
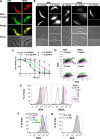
Tryparedoxin peroxidases, distant relatives of glutathione peroxidase 4 in higher eukaryotes, are responsible for the detoxification of lipid-derived hydroperoxides in African trypanosomes. The lethal phenotype of procyclic Trypanosoma brucei that lack the enzymes fulfils all criteria defining a form of regulated cell death termed ferroptosis. Viability of the parasites is preserved by α-tocopherol, ferrostatin-1, liproxstatin-1 and deferoxamine. Without protecting agent, the cells display, primarily mitochondrial, lipid peroxidation, loss of the mitochondrial membrane potential and ATP depletion. Sensors for mitochondrial oxidants and chelatable iron as well as overexpression of a mitochondrial iron-superoxide dismutase attenuate the cell death. Electron microscopy revealed mitochondrial matrix condensation and enlarged cristae. The peroxidase-deficient parasites are subject to lethal iron-induced lipid peroxidation that probably originates at the inner mitochondrial membrane. Taken together, ferroptosis is an ancient cell death program that can occur at individual subcellular membranes and is counterbalanced by evolutionary distant thiol peroxidases VSports手机版. .
Keywords: Trypanosoma brucei; cell biology; ferroptosis; trypanothione; tryparedoxin peroxidase V体育安卓版. .
© 2018, Bogacz et al.
"VSports最新版本" Conflict of interest statement
MB, RK No competing interests declared
Figures

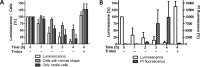
VSports在线直播 - References
-
- Budde H, Flohé L, Hecht HJ, Hofmann B, Stehr M, Wissing J, Lünsdorf H. Kinetics and redox-sensitive oligomerisation reveal negative subunit cooperativity in tryparedoxin peroxidase of Trypanosoma brucei brucei. Biological Chemistry. 2003;384:619–633. doi: 10.1515/BC.2003.069. - DOI (V体育平台登录) - PubMed
"VSports" Publication types
- "V体育官网" Actions
V体育官网 - MeSH terms
- VSports - Actions
- "VSports注册入口" Actions
- "V体育ios版" Actions
- VSports - Actions
- "VSports最新版本" Actions
- "V体育平台登录" Actions
- "V体育2025版" Actions
- Actions (VSports手机版)
- "VSports手机版" Actions
- "V体育官网" Actions
Substances
- V体育官网 - Actions
- "V体育ios版" Actions
VSports在线直播 - LinkOut - more resources
Full Text Sources
V体育安卓版 - Other Literature Sources
Medical
