V体育安卓版 - Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer
- PMID: 29157287
- PMCID: "VSports" PMC5697069
- DOI: 10.1186/s40425-017-0298-2
Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer
Abstract
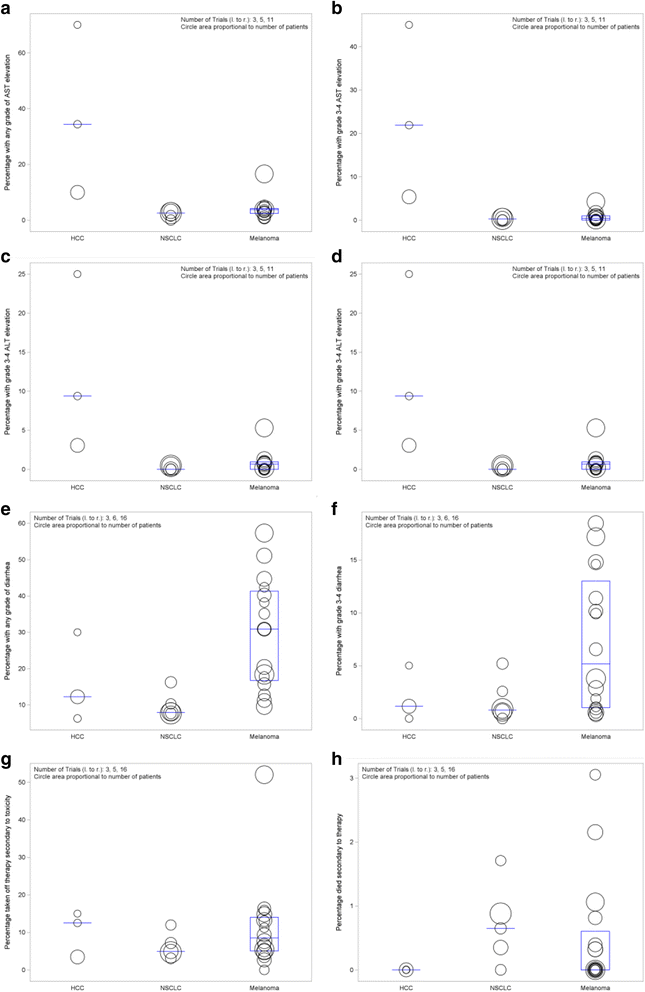
Background: Hepatocellular carcinoma (HCC) is a major health problem worldwide with increasing incidence rates. As HCC traditionally occurs in chronically inflamed livers, this inflammation aids to drive oncogenesis and often renders these lesions to be immunogenic and therefore potential targets for immunotherapy. As patients with HCC generally have underlying liver dysfunction, we sought to determine if immune checkpoint inhibitors were safe to use in patients with HCC as compared to melanoma and non-small cell lung cancer (NSCLC) in terms of the gastrointestinal side effects of elevation of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and diarrhea as well as patients who drop out of the study due to drug toxicity and death secondary to drug toxicity. VSports手机版.
Methods: A literature review was performed for clinical trials that have been completed with single agent immune checkpoint inhibitors for patients with HCC, melanoma, and NSCLC. Gastrointestinal related adverse events including elevation of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and diarrhea were analyzed as well as those patients who were taken off therapy secondary to drug related toxicity and patients who died as a result of therapy. V体育安卓版.
Results: We found that although patients with HCC treated with immune checkpoint inhibitors have a substantial increase in AST/ALT as compared to patients with melanoma and NSCLC, this does not cause the patients to come off therapy or cause death secondary to drug toxicity V体育ios版. .
Conclusions: We propose immune checkpoint inhibitors are safe to pursue in the treatment of HCC. VSports最新版本.
Keywords: Adverse events; Hepatocellular carcinoma; Immune checkpoint inhibitors; Immunotherapy V体育平台登录. .
Conflict of interest statement
Ethics approval and consent to participate
Not applicable, no patient consent or ethical approval was required as this analysis was performed with preexisting published data and no patients or animals were subject to harm.
Consent for publication
Not applicable
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, et al. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. - DOI - PMC - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008, 359:378-390. - PubMed
-
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. - DOI - PubMed
MeSH terms
- Actions (V体育官网入口)
- V体育安卓版 - Actions
- Actions (VSports最新版本)
- V体育2025版 - Actions
- "VSports" Actions
- Actions (V体育ios版)
- Actions (VSports app下载)
- V体育官网入口 - Actions
Substances
LinkOut - more resources
VSports最新版本 - Full Text Sources
Other Literature Sources
Medical
Research Materials
