Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages
- PMID: 28392147
- PMCID: V体育官网入口 - PMC5467448
- DOI: 10.1016/j.chembiol.2017.03.009
Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages
"V体育官网入口" Abstract
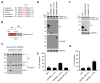
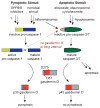
Pyroptosis is a lytic form of programmed cell death mediated by the inflammatory caspase-1, -4, and -5. We recently discovered that small-molecule inhibitors of the serine peptidases DPP8 and DPP9 (DPP8/9) induce pro-caspase-1-dependent pyroptosis in monocytes and macrophages. Notably, DPP8/9 inhibitors, unlike microbial agents, absolutely require caspase-1 to induce cell death. Therefore, DPP8/9 inhibitors are useful probes to study caspase-1 in cells. Here, we show that, in the absence of the pyroptosis-mediating substrate gasdermin D (GSDMD), caspase-1 activates caspase-3 and -7 and induces apoptosis, demonstrating that GSDMD is the only caspase-1 substrate that induces pyroptosis. Conversely, we found that, during apoptosis, caspase-3/-7 specifically block pyroptosis by cleaving GSDMD at a distinct site from the inflammatory caspases that inactivates the protein VSports手机版. Overall, this work reveals bidirectional crosstalk between apoptosis and pyroptosis in monocytes and macrophages, further illuminating the complex interplay between cell death pathways in the innate immune system. .
Keywords: DPP8/9 inhibitors; apoptosis; caspase-1; caspase-3; caspase-7; cell death; gasdermin D; macrophages; monocytes; pyroptosis V体育安卓版. .
Copyright © 2017 Elsevier Ltd V体育ios版. All rights reserved. .
Figures


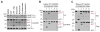
"VSports在线直播" References
-
- Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C, Butchar J, et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009;5:e1000361. - "VSports最新版本" PMC - PubMed
-
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. - PubMed
-
- Broz P. Immunology: Caspase target drives pyroptosis. Nature. 2015;526:642–643. - PubMed
MeSH terms
- VSports app下载 - Actions
- "V体育平台登录" Actions
- "VSports app下载" Actions
- "V体育平台登录" Actions
- "VSports最新版本" Actions
- Actions (VSports注册入口)
- "VSports最新版本" Actions
- "VSports app下载" Actions
- V体育官网入口 - Actions
- "VSports注册入口" Actions
- "V体育官网" Actions
- V体育平台登录 - Actions
- VSports最新版本 - Actions
Substances
- "VSports" Actions
- VSports app下载 - Actions
- V体育官网入口 - Actions
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- Actions (V体育ios版)
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
V体育官网入口 - Research Materials

