V体育ios版 - Programmed cell death as a defence against infection
- PMID: 28138137
- PMCID: PMC5328506
- DOI: "V体育官网" 10.1038/nri.2016.147
Programmed cell death as a defence against infection
Abstract
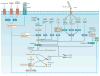
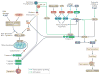
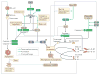
Eukaryotic cells can die from physical trauma, which results in necrosis. Alternatively, they can die through programmed cell death upon the stimulation of specific signalling pathways. In this Review, we discuss the role of different cell death pathways in innate immune defence against bacterial and viral infection: apoptosis, necroptosis, pyroptosis and NETosis. We describe the interactions that interweave different programmed cell death pathways, which create complex signalling networks that cross-guard each other in the evolutionary 'arms race' with pathogens. Finally, we describe how the resulting cell corpses - apoptotic bodies, pore-induced intracellular traps (PITs) and neutrophil extracellular traps (NETs) - promote the clearance of infection VSports手机版. .
Conflict of interest statement
The authors declare no competing interests.
V体育平台登录 - Figures

References
-
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. - PMC (VSports最新版本) - PubMed
-
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. - "V体育平台登录" PubMed
-
- Vanden Berghe T, Hassannia B, Vandenabeele P. An outline of necrosome triggers. CMLS, Cell Mol Life Sci. 2016;73:2137–2152. - V体育官网入口 - PMC - PubMed
-
- Dillon CP, Tummers B, Baran K, Green DR. Developmental checkpoints guarded by regulated necrosis. CMLS, Cell Mol Life Sci. 2016;73:2125–2136. - PMC (V体育官网) - PubMed
Publication types
MeSH terms
- VSports app下载 - Actions
- Actions (V体育官网)
"V体育官网" Grants and funding
"V体育ios版" LinkOut - more resources
Full Text Sources
Other Literature Sources

