Abstract
Tumor necrosis factor (TNF) is a master pro-inflammatory cytokine, and inappropriate TNF signaling is implicated in the pathology of many inflammatory diseases. Ligation of TNF to its receptor TNFR1 induces the transient formation of a primary membrane-bound signaling complex, known as complex I, that drives expression of pro-survival genes. Defective complex I activation results in induction of cell death, in the form of apoptosis or necroptosis. This switch occurs via internalization of complex I components and assembly and activation of secondary cytoplasmic death complexes, respectively known as complex II and necrosome V体育平台登录. In this review, we discuss the crucial regulatory functions of ubiquitination—a post-translational protein modification consisting of the covalent attachment of ubiquitin, and multiples thereof, to target proteins—to the various steps of TNFR1 signaling leading to necroptosis.
Keywords: Ubiquitination, Necroptosis, TNFR1, RIPK1, c IAP1/2, LUBAC, A20, CYLD
Introduction
Host defense against invading microbes is mediated by the selective sensing of some of their conserved components by an array of innate immune receptors that are mainly expressed by cells of the immune system and barrier cells that line the outside world V体育官网入口. Recognition of these so-called pathogen-associated molecular patterns (PAMPs) by the pattern recognition receptors (PRRs) leads to the activation of signaling pathways [including the mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways] that collectively drive the production and release of pro-inflammatory cytokines and chemokines [1]. These cytokines can modulate the innate immune response by binding to different plasma membrane receptors, including members of the tumor necrosis factor receptor superfamily (TNFR-SF). In addition to their ability to further induce inflammatory mediators, some TNFR-SF members known as death receptors (DRs) possess a cytoplasmic death domain (DD) that allows them to transduce a regulated pro-death signal resulting in apoptotic or necroptotic death of the cell [2]. The combination of inflammatory cytokine production and activation of cell death pathways alerts the immune system and clears the potentially harmful microbes from the organism. Although crucial for the protection of the organism from microbial insult, the inflammation response needs tight regulation because inappropriate inflammatory signals and excessive cell death are at the origin of various pathologies.
Ubiquitination, a post-translational modification of proteins consisting in the covalent attachment of the small 8 kDa protein ubiquitin (Ub), and multiples thereof, to target proteins, plays a crucial role in the regulation of various aspects of the inflammatory response [3]. Ubiquitination is a dynamic process that is catalyzed by the action of a three-step enzymatic cascade involving a Ub-activating enzyme (E1), a Ub-conjugating enzyme (E2) and a Ub-ligase (E3), and which is negatively regulated by de-ubiquitinases (DUBs). Ubiquitin is first loaded on the E1 in an ATP-dependent manner. Next, Ub is discharged from the E1 and transferred to the E2. The E2 can then bind to an E3 that mediates the transfer of Ub to a lysine (K) residue of a substrate via an isopeptide bond. The attachment of a single moiety of Ub to a target protein is referred to as mono-ubiquitination. Since Ub itself contains seven lysine residues (K6, K11, K27, K29, K33, K48 and K63) that can all serve as acceptor sites for another Ub molecule, ubiquitination can also result in the conjugation of at least seven different poly-Ub chains to a substrate. An additional chain, known as the M1-linked poly-Ub chain (also called linear Ub chain) can be generated via a peptide bond between the N-terminal methionine (M1) of one Ub to the C-terminal glycine of another [4–6]. The Linear UBiquitin chain Assembly Complex (LUBAC) is the only E3 identified so far capable of exclusively generating these M1-linked ubiquitin linkages. Because the internal lysines of Ub are present in different positions on the surface of the protein, each of the eight possible Ub linkages adopts a structurally distinct conformation [7] VSports在线直播. Proteins harboring Ub-binding domains (UBDs) specifically recognize these structurally different Ub linkages, and thereby translate the Ub code into distinct cellular functions [8]. De-ubiquitinases terminate or modulate the Ub-dependent signal by removing the Ub moieties from the substrates. Two major classes of DUBs exist: substrate-specific DUBs and linkage-specific DUBs [9]. The specificity of the former class is regulated by substrate recognition and the enzymes that constitute that group are usually able to cleave any type of linkage. Most members of the Ub-specific protease (USP) family are considered substrate-specific DUBs. In contrast, the other families of DUBs are mainly linkage-specific and are able to process only few specific types of Ub linkages. For both DUB types, substrate specificity can be further regulated by the presence of additional UBDs, the binding to UBD-containing adaptor proteins and post-translational modifications [9].
It is evident that the spatiotemporal regulation of the expression and/or activity of all these different types of Ub-modulating enzymes tightly controls the intracellular Ub code and, as a consequence, also the cellular response to a specific trigger. Indeed, interfering with E3s and DUBs greatly affects the responses activated downstream of several innate immune receptors. In this review, we focus on the TNF signaling pathway and discuss the literature on the role of ubiquitination in the regulation of TNF-mediated necroptosis, a caspase-independent regulated form of necrosis. During this cell death process, and in contrast to apoptosis, the cell and its organelles swell until this process finally culminates in plasma membrane rupture and cell death. TNF-mediated necroptosis relies on RIPK1 kinase-dependent assembly of a cytosolic death complex, known as the necrosome, whose core components apart from RIPK1 are RIPK3 and MLKL [10–13]. The necrosome presumably originates from the dissociation of a primary plasma membrane-associated signaling complex, called TNFR1 complex I or TNRF1-SC, which forms within seconds following engagement of TNFR1 by TNF [14, 15]. It is therefore important to start our review by describing the role of ubiquitination in the regulation of TNFR1 complex I assembly and function V体育2025版.
Role of poly-ubiquitination in regulating TNFR1 complex I assembly
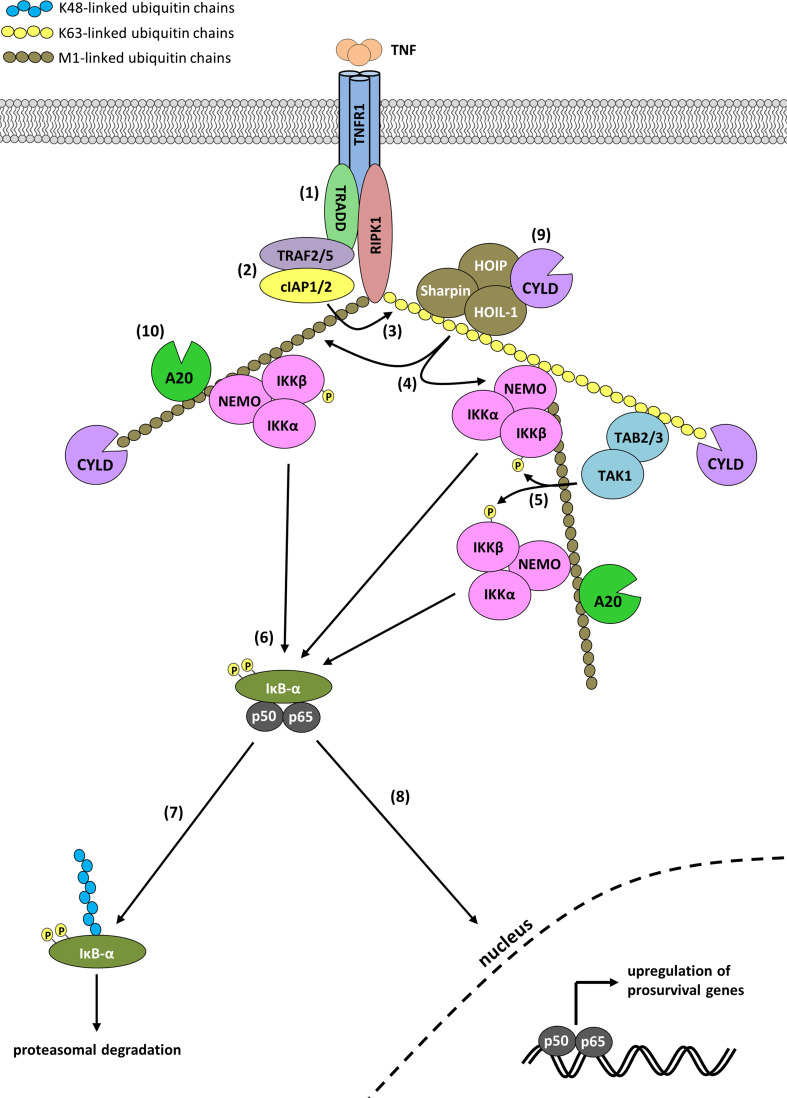
Binding of TNF to TNFR1 induces the independent recruitment of the adaptor protein TRADD and the kinase RIPK1 to the receptor’s DD via homotypic DD interactions (Fig. 1). Complex I-recruited TRADD serves as platform for recruitment of TRAF2 and/or TRAF5. The cIAP interaction motif (CIM) contained within TRAF2 allows recruitment of two closely related members of the Inhibitor of Apoptosis Protein (IAP) family, the Ub E3s cIAP1 and cIAP2 [16, 17]. Following their recruitment, cIAP1/2 ubiquitinate components of complex I, such as RIPK1 and cIAP1 itself, with K63-, K11- and K48-linked poly-Ub chains [18–23]. The Ub chains generated by cIAP1/2 in turn allow the subsequent recruitment of LUBAC, a Ub E3 complex consisting of the central catalytic component HOIP (RNF31), HOIL-1 (RBCK1) and SHARPIN (SIPL1) that exclusively generates M1-linked poly-Ub chains [17, 23–26]. LUBAC adds M1-linked Ub chains to components of complex I, including RIPK1, NEMO, TRADD and TNFR1 itself [17, 23, 26–28]. The Ub chains placed on components of complex I by cIAP1/2 and LUBAC serve as scaffold for recruitment and retention of the TAB 2/3/TAB 1/TAK1 and NEMO/IKKα/IKKβ kinase complexes (Fig. 1). Recruitment of these complexes to the Ub chains is mediated by the UBDs present in TAB 2/3 and NEMO, respectively [29–32]. Whereas TAB 2/3 specifically binds K63-linked poly-Ub chains, NEMO binds to M1-, K63-, and K11-linked chains, yet with approximately 100-fold higher affinity to M1-linked over K63- and K11-linked chains [22, 30, 32–35]. Following activation, TAK1 activates the downstream MAPKs (JNK, p38 and ERK) and IKKβ by phosphorylation. IKKβ in turn phosphorylates IκBα, which leads to its K48-linked poly-ubiquitination by the Skp1-Cullin-F-box (SCF)/β-TRCP E3 complex and results in proteasomal degradation of IκBα [36–39]. This liberates the p50/RelA NF-κB dimer from IκBα-imposed inhibition, and allows p50/RelA to translocate to the nucleus where it drives expression of NF-κB target genes (Fig. 1). The Ub-dependent signaling emerging from complex I is negatively regulated by DUBs that mediate disassembly of complex I by hydrolyzing the Ub modifications present in the complex. Some DUBs, such as A20 and Cezanne, are upregulated by TNF-induced NF-κB and reported to be part of a negative feed-back mechanism aimed at repressing NF-κB activation [40, 41]. A20 was initially proposed to repress RIPK1-mediated NF-κB activation by functioning as a DUB removing the K63-linked Ub chains from RIPK1, and as an E3 conjugating RIPK1 with K48-linked Ub chains promoting its proteasomal degradation [41]. Several findings are now questioning the validity of this model, such as the linkage specificity of its DUB activity and, more importantly, the fact that mice harboring mutations affecting A20′s DUB or E3 activities do not develop any signs of inflammation and are grossly normal [42–45]. Other DUBs, such as CYLD and USP21, are constitutively expressed and also implicated in regulating TNFR1 signaling [46–49]. It was recently demonstrated that CYLD is recruited to complex I via direct binding to HOIP independently of LUBAC activity, and that A20 directly binds to M1-linked Ub chains in complex I, therefore requiring the E3 activity of LUBAC for recruitment [28]. Once recruited, CYLD limits NF-κB activation by removing K63-linked and M1-linked poly-Ub chains on several components of complex I, including RIPK1 [28, 47–50]. Interestingly, CYLD and A20 seem to have opposing effect on M1-linked poly-Ub chain stability VSports. Whilst CYLD degrades them, A20 binds to them, thereby preventing their cleavage and, hence, removal (Fig. 1) [28]. Although the specific roles of all the individual DUBs in complex I-dependent signaling are not yet fully understood, the targeted recruitment of individual DUBs to particular linkage types, together with their specificity in hydrolyzing specific Ub linkages, likely serves to fine-tune the precise extent and duration of signaling. .
Fig. 1.
TNFR1 complex I assembly and NF-κB activation. TNF ligation to trimeric TNFR1 leads to recruitment of TRADD and RIPK1 (1). TRAF2/5 and cIAP1/2 are then recruited to TRADD (2), which allows cIAP1/2 to conjugate RIPK1 with Ub chains, including K63-linked chains (3). The Ub chains added to RIPK1 then allow further recruitment of the TAK1, IKK and LUBAC complexes via the respective Ub binding domains of TAB 2/3, NEMO and HOIL/Sharpin. Once recruited, LUBAC adds M1-linked Ub chains to several complex I components, including RIPK1 and NEMO. This leads to the recruitment of additional IKK complexes (4). Activated TAK1 then activates IKKα/IKKβ by phosphorylation (5). IKKβ subsequently phosphorylates IκBα at Ser32 and Ser36 (6) thereby marking it for K48-linked ubiquitination and subsequent proteasomal degradation (7). Released from IκBα inhibition, the p50/p65 NF-κB transcription factor translocates to the nucleus to induce expression of several pro-survival genes (8). Ubiquitination in TNFR1 complex I is negatively regulated by LUBAC-recruited CYLD, which removes both K63- and M1-linked Ub chains from several substrates, including RIPK1 (9). In contrast, binding of A20 to M1-linked chains stabilizes them by preventing their degradation (10) VSports app下载.
"V体育2025版" Role of poly-ubiquitination in regulating the two TNFR1 complex I-dependent cell death checkpoints
TNF-induced necroptosis is not the default cell death pathway activated by TNFR1. Indeed, necroptosis only occurs when caspase-8 activation fails or is inhibited, indicating that caspase-8-mediated apoptosis actively represses necroptosis [51–55]. Since any condition that sensitizes to TNF-induced apoptosis will therefore also sensitize to TNF-induced necroptosis when caspase-8 is additionally inhibited, understanding how ubiquitination regulates caspase-8 activation is therefore of great relevance to comprehend TNF-induced necroptosis V体育官网.
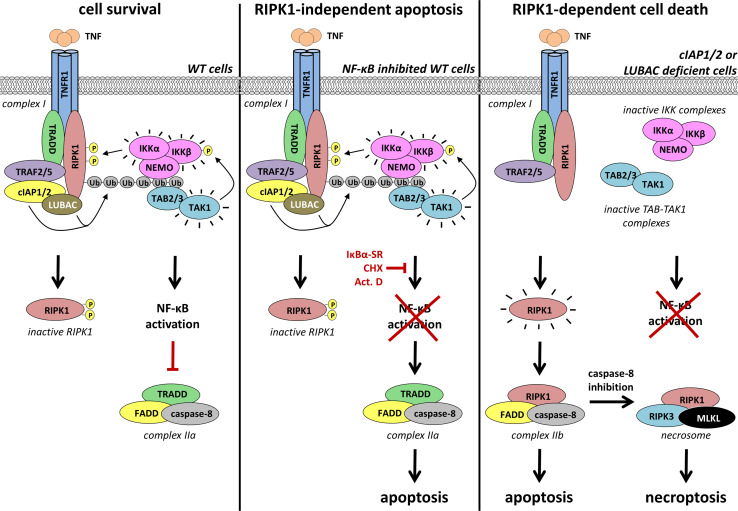
In most cases, TNFR1 engagement is insufficient to kill cells but instead results in the ubiquitination-dependent activation of gene induction through the NF-κB and MAPK pathways (Fig. 2). However, when the NF-κB-dependent response is inhibited, such as upon genetic deletion of NF-κB, expression of a dominant-negative form of IκBα or in the presence of transcriptional (actinomycin D) or translational (cycloheximide) inhibitors, cells succumb to TNFR1 activation by TNF [56, 57]. Under these circumstances, the switch from a pro-survival to a pro-death response involves the formation of a cytoplasmic caspase-8-activating complex, known as TNFR1 complex II (Fig. 2) [58, 59]. Complex II assembles when TRADD dissociates from complex I and engages FADD in the cytosol [14]. FADD in turn serves as a platform for the recruitment and activation of caspase-8, resulting in apoptotic cell death [60]. The transient formation of complex II normally does not result in cell death since complex I–dependent transcriptional upregulation of pro-survival genes such as FLIP counteracts death induction from complex II. FLIP is highly homologous to caspase-8 but lacks catalytic activity and competes with caspase-8 for recruitment to FADD in TNF complex II, thereby preventing full caspase-8 activation.
Fig. 2.
Regulation of the different TNFR1 cell death checkpoints by poly-ubiquitination. Left panel cIAP1/2- and LUBAC-mediated ubiquitination of TNFR1 complex I components activates the IKK complex. Active IKKα/β then promote cell survival by NF-κB-dependent upregulation of pro-survival genes that counteract the activation of the cytosolic apoptosis-inducing complex IIa (TRADD-FADD-caspase-8). In addition, IKKα/β directly phosphorylate RIPK1 in complex I thereby preventing RIPK1 activation and, as a consequence, RIPK1-mediated cell death (apoptosis and necroptosis). Middle panel inhibition of the NF-κB pathway downstream of the IKK complex, i.e. by an undegradable IκBα (IκBα-SR), the transcription inhibitor actinomycin D (Act. D) or the translation inhibitor cycloheximide (CHX), induces complex IIa-dependent apoptosis. In this scenario, the active IKK complex still prevents RIPK1 activation and RIPK1-mediated cell death (apoptosis and necroptosis). Right panel cIAP1/2 or LUBAC deficiency impairs TNF-induced activation of the IKK complex. As a result, the IKK-mediated brake on RIPK1 is relieved. Active RIPK1 then promotes assembly of complex IIb (RIPK1-FADD-caspase-8) resulting in RIPK1-dependent apoptosis. Inhibition of caspase-8 shifts the cell death modality from RIPK1-dependent apoptosis to RIPK1-dependent necroptosis, induced by the necrosome (RIPK1/RIPK3/MLKL)
It has now become clear that the NF-κB-dependent induction of pro-survival genes is not the only cell death checkpoint regulated by complex I, and that the role of ubiquitination in preventing TNF-mediated cell death exceeds canonical NF-κB activation [61]. Indeed, when ubiquitination events in complex I are perturbed by the absence of the E3 ligases cIAP1/2 or LUBAC, cells also die after TNF stimulation due to increased formation of complex II [19, 58, 62–66]. In this case, complex II formation is highly dependent on RIPK1 and its kinase activity. It is thought that the absence of cIAP1/2 or LUBAC results in insufficient ubiquitination of RIPK1 in complex I, which results in RIPK1 promoting the formation of complex II and cell death. (Fig. 2) [59]. In contrast with complex II-mediated apoptosis induced by inhibiting the NF-kB response downstream, which occurs independently of RIPK1, the kinase activity of RIPK1 is crucial for complex II assembly and apoptosis induction in the absence of cIAP1/2 or LUBAC [58, 67, 68]. Therefore, to distinguish these two different modes of inducing complex II, the former is also called complex IIa, and the latter complex IIb. In addition to apoptosis, deficiency in cIAP1/2 or LUBAC also sensitizes cells to TNF-induced necroptosis that is dependent on the kinase activity of RIPK1 [11, 23, 64, 69, 70]. These findings indicated that cIAP1/2 and LUBAC negatively regulate the pro-death function of RIPK1 on top of promoting canonical NF-κB activation. The Ub chains conjugated to RIPK1 by cIAP1/2 and LUBAC are therefore not only required to activate the canonical NF-κB pathway but also to repress RIPK1 kinase-dependent death (Fig. 2). This concept is supported by the fact that cells expressing a form of RIPK1 that is mutated for its Ub acceptor site (K377R) undergo RIPK1-dependent death following TNFR1 engagement by TNF [31, 71]. Further supporting the notion that cIAP1/2 regulate the pro-death function of RIPK1 directly is the formation of the ‘ripoptosome’, a RIPK1-dependent caspase-8-activating complex similar to complex II. However, in contrast to complex II, it forms spontaneously (independently from death receptors) when cIAP1/2 are depleted [72, 73].
The importance of cIAP1/2- and LUBAC-mediated ubiquitination in preventing uncontrolled RIPK1 activation and consequent cell death has also been elegantly demonstrated in a number of genetic mouse models. While the genetic deletion of the catalytic component of LUBAC, HOIP, is embryonically lethal at day E10.5, a null mutation in the Sharpin gene is not lethal but instead results in the development of a severe multi-organ inflammatory phenotype called chronic proliferative dermatitis (cpdm) [64, 74, 75]. These cpdm mice display severe inflammation in the skin, liver, gut, lung and oesophagus, together with a loss of Peyer’s patches and splenomegaly [74, 76]. The inflammatory phenotype of cpdm mice is driven by aberrant TNFR1-mediated cell death [23, 65, 77]. Interestingly, the cpdm phenotype can also be completely prevented by crossing with RIPK1 kinase-dead knockin mice as well as with the combination of RIPK3 deficiency and caspase-8 heterozygosity [65, 66]. These genetic studies confirm the role of LUBAC-mediated ubiquitination in repressing RIPK1 pro-death function and demonstrate that in the absence of fully functional LUBAC, the kinase activity of RIPK1 can both induce apoptosis and necroptosis in vivo. The role of cIAP1/2 in vivo has been more difficult to study as the single knock-outs do not show any overt phenotype whereas the double knock-out, similar to the HOIP−/− mice, causes embryonic lethality at day E10.5 due to cardiovascular failure as a consequence of TNFR1-driven yolk sac endothelial cell death [63, 78, 79]. Dysregulation of RIPK1 in these knockouts has also been shown genetically since deletion of RIPK1 slightly delays the lethality of cIAP1−/− cIAP2−/− animals [63]. However, RIPK1 deficiency on its own causes early postnatal lethality by uncontrolled caspase-8-mediated apoptosis and RIPK3-mediated necroptosis [80–82]. It would therefore be interesting to test whether replacing wild-type RIPK1 by a kinase-dead RIPK1 via gene knockin could prevent embryonic lethality of cIAP1/2 or HOIP deficiency as this would impair RIPK1’s pro-death function whilst keeping its pro-survival scaffold function intact.
Importantly, CYLD repression was shown to protect cells from TNF-mediated RIPK1 kinase-dependent apoptosis and necroptosis, which additionally supports the notion that the K63- and M1-linked poly-Ub chains added to complex I components by cIAP1/2 and LUBAC prevent RIPK1 from initiating the formation of the cytosolic death complex II [28, 50, 58, 67, 70]. Apart from functioning as an inhibitor of the canonical NF-κB pathway, A20 is also known as a potent inhibitor of TNF-induced apoptosis and necroptosis, but the mechanism accounting for this function had remained unclear [69, 83, 84]. The recent finding that A20 protects M1-linked poly-Ub chains from CYLD-mediated degradation in complex I now provides a crucial element in our understanding of the cell-protective function of A20 [28].
It was initially believed that the Ub chains conjugated to RIPK1 directly prevent RIPK1 from integrating into complex IIb or the necrosome. More recent findings also suggest that not only the Ub chains themselves, but also the kinases recruited to them control RIPK1 kinase-dependent death. Indeed, IKKα/IKKβ were shown to prevent RIPK1 from integrating into complex IIb through direct phosphorylation in complex I, thereby suggesting a model according to which IKKα/IKKβ directly repress RIPK1 kinase activity (Fig. 2) [85]. In accordance with this model, any conditions affecting IKKα/IKKβ activation downstream of RIPK1 ubiquitination, such as deficiency in TAK1, NEMO or IKKα/IKKβ, were shown to sensitize cells to RIPK1 kinase-dependent apoptosis and necroptosis without affecting RIPK1 ubiquitination in complex I [67, 85–89]. Importantly, this role of IKKα/IKKβ was demonstrated to be independent of NF-κB activation.
Direct control of the necrosome by poly-ubiquitination
Necroptosis induction was initially reported to occur following recruitment of RIPK3 to a RIPK1/FADD/caspase-8 complex, suggesting that the necrosome originates from an inactive complex IIb. More recent papers however suggested a model in which an independent RIPK1/RIPK3/MLKL complex is formed in parallel to complex IIb [12]. The precise relation between complex IIb and the necrosome is therefore currently unclear and additional work is required to better define whether they represent two independent complexes or variations of the same complex.
As mentioned in the previous section, poly-ubiquitination plays crucial roles in regulating necroptosis by allowing complex I assembly and fine-tuning the two complex-I-dependent cell death check points, namely the NF-κB-dependent induction of pro-survival factors and the NF-κB-independent contribution of RIPK1 to the cell demise. Recent studies suggest that poly-ubiquitination also plays a regulatory role at the level of the cell death-inducing complex itself (Fig. 3). Indeed, TNFR1-induced complex IIb/necrosome-associated components have been reported to show post-translational modifications reminiscent of ubiquitination [67, 90–93]. Necrosome-associated RIPK3 is conjugated with K63-linked poly Ub chains [91], whereas RIPK1 has been reported to contain both M1- and K63-linked Ub chains [90, 93]. It is currently unclear whether these ubiquitin chains regulate the cell death outcome in a positive or negative manner, and whether they additionally regulate a non-cell death signaling pathway emanating from this complex. It appears that only a fraction of the complex IIb/necrosome-associated RIPK1 (and RIPK3) is ubiquitinated, whereas all of the RIPK1 associated with TNFR1 complex I is ubiquitinated [67]. This difference in the amount of ubiquitinated RIPK1 molecules in both complexes may question the importance of these post-translational modifications in the regulation of the cell death complex itself. Indeed, it was demonstrated that HOIP knockdown partially affects RIPK1 ubiquitination in the necrosome, while not affecting necroptotic cell death significantly [93]. The identity of the E3s ubiquitinating the different components of the necrosome are still under debate, and it is not entirely clear whether the poly-Ub chains conjugated to RIPK1 originate from its ubiquitination in TNFR1 complex I. RIPK1 ubiquitination in the necrosome was reported to occur independently of cIAP1/2 and only partially depending on HOIP, which would suggest implication of additional E3s [93]. In vitro studies have shown that cIAP1/2 are able to add different types of poly-Ub chains to RIPK3, yet the relevance of this observation for necroptotic signaling is not established [94]. TRAF2 was also shown to bind to MLKL in cells and was proposed to suppress TNF-induced necroptosis by preventing MLKL to exert its pro-death function [95]. Nevertheless, although MLKL was reported to be ubiquitinated in cells, the suppressive function of TRAF2 appears to occur independently of its supposed activity as a Ub E3 [95, 96].
VSports最新版本 - Fig. 3.
Direct control of the necrosome by poly-ubiquitination. Activated RIPK3 in the necrosome induces MLKL activation by phosphorylation. Active MLKL then translocates to phosphatidyl inositol phosphates in the plasma membrane. Plasma-membrane associated MLKL oligomerizes into high-molecular weight complexes and dysregulates the cellular ion homeostasis resulting in cell swelling and plasma membrane rupture. Although the functional relevance is not entirely clear at this time, RIPK1 and RIPK3 are reported to be poly-ubiquitinated in the necrosome, presumably independently of ubiquitination in TNFR1 complex I. RIPK1 is associated with both M1-linked and K63-linked ubiquitin chains while only K63-linked Ub chains have been described for RIPK3. LUBAC and cIAP1/2 have been involved in the conjugation of these Ub chains. From the DUB point of view, CYLD is reported to promote necroptosis induction by removing the K63-linked Ub chains on RIPK1. In contrast, A20 prevents necroptosis induction by removing the K63-linked Ub chains on RIPK3
Poly-ubiquitination at the level of the necrosome was also demonstrated in studies making use of cells depleted of the DUBs CYLD and A20. CYLD deficiency greatly protects against TNF-induced necroptosis and this protection correlates with an increase in RIPK1 poly-ubiquitination [28, 53, 70, 90]. Indeed, CYLD can hydrolyze M1- and K63-linked Ub chains conjugated to RIPK1 [28, 47–50]. Interestingly, Chan and colleagues showed that CYLD-deficiency also affects ubiquitination of RIPK1 in the necrosome, which implies a role of CYLD in arming RIPK1 in both, complex I and the necrosome [90]. In contrast to CYLD, A20-deficient cells are strongly sensitized to TNF-induced necroptosis and a recent study associates this sensitization to increased RIPK3 poly-ubiquitination in the necrosome [91]. In support of this interpretation, the authors showed that mutation of Lys5 in RIPK3 partially inhibited necrosome-associated RIPK3 poly-ubiquitination and TNF-induced necroptotic cell death [91]. It is also of interest to note that absence of A20 does not only increase the amount of poly-ubiquitinated, but also of non-ubiquitinated, RIPK3 in the necrosome. This may be the result of an increased stability of the necrosome in presence of poly-ubiquitinated RIPK3, or reflective of the more upstream role of A20 in the pathway. Indeed, A20 protects M1-linked poly-Ub chains in complex I and thereby prevents RIPK1 from initiating the formation of the cytosolic death-inducing complex II [28].
It is intriguing to note that ubiquitination of RIPK1 and RIPK3 in the necrosome is reported to have opposite effect on necroptosis induction. Those post-translational modifications would repress RIPK1 but activate RIPK3. Future work on the identification of the type of Ub chains conjugated to each protein is clearly needed to provide a molecular basis for the better understanding of these apparent opposing consequences. Also, uncoupling complex I from necrosome assembly may help evaluating the respective contribution of the different E3s and DUBs to the two different steps of the pathway. It might, for example, be interesting to study how the presence or absence of these enzymes affects necroptosis induced by forced homo- and heterodimerization of RIPK1 and RIPK3.
"VSports手机版" Concluding remarks
In recent years, great advances have been made in the understanding of the molecular mechanisms of TNFR1-induced necroptosis. However, the exact consequences, functions and interconnections between the diverse post-translational modifications regulating this signaling pathway still remain to be investigated in more detail. Although ubiquitination has clearly emerged as a major regulatory mechanism, the identity of all the substrates, the exact linkage composition of the different poly-Ub chains, the precise roles of the diverse Ub-related enzymes and their specificity for complex I vs. complex II/necrosome still require better understanding. Most of the knowledge acquired over the past years has been generated by the use of cutting-edge biochemistry, often combined with sophisticated in- vivo models employing component-deficient mice and/or cells. It is now important to understand how the activities of the ubiquitination-modifying enzymes are regulated under physiological conditions and how this is deregulated in pathological conditions. It is for example known that binding of TNF to TNFR2 induces degradation of a pool of TRAF2/cIAP1/cIAP2 that consequently affects ubiquitination in TNFR1 complex I and switches the TNFR1-mediated response from survival to death [97]. Co-stimulation with other members of the TNFR superfamily can exert similar effects. What are, however, the conditions that regulate other ubiquitination-modifying enzymes? A possible hint comes from the fact that necroptosis is often associated with the generation of ROS and that the activity of A20 and other OTU deubiquitinases was shown to be regulated by reversible oxidation [98]. Also, viral infection can trigger necroptosis and some viruses have been reported to interfere with the enzymatic activities of endogenous E3s and DUBs [99, 100].
RIPK1 plays a pivotal role in determining the cellular response to TNF. While it functions as a central signaling platform in the TNFR1 complex I to drive NF-κB- and MAPK-mediated cell survival and inflammation, it also plays a crucial role in the cytosolic complex II and, consequently, in the induction of cell death. In accordance, ubiquitination of RIPK1 in both complexes is a key event in regulating the TNFR1 signaling pathway to necroptosis. Nevertheless, necroptosis induced by receptors other than TNFR1, such as TLR3 and DAI, does not require RIPK1. Downstream of these receptors, the RHIM domain required to recruit and activate RIPK3 is respectively provided by TRIF or DAI itself [101–104]. Studying the role of E3s and such as cIAP1/2 and LUBAC as well as DUBs such as A20 and CYLD in the ubiquitination-dependent regulation of necroptosis downstream of these receptors are therefore interesting perspectives.
Acknowledgments (VSports app下载)
MJMB has a tenure track position within the Multidisciplinary Research Program of Ghent University (GROUP-ID).
Footnotes (VSports最新版本)
Y. Dondelinger and M. Darding contributed equally to this work.
M. J. M. Bertrand and H. Walczak are co-senior and co-corresponding authors.
Contributor Information
Mathieu J. M. Bertrand, Phone: +3293313720, Email: mathieu.bertrand@qiuluzeuv.cn
Henning Walczak, Phone: +4476796452, Email: h.walczak@qiuluzeuv.cn.
"V体育2025版" References
- 1.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 2.Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14(11):727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 3.Zinngrebe J, Montinaro A, Peltzer N, Walczak H. Ubiquitin in the immune system. EMBO Rep. 2014;15(1):28–45. doi: 10.1002/embr.201338025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, Sano S, Tokunaga F, Tanaka K, Iwai K. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25(20):4877–4887. doi: 10.1038/sj.emboj.7601360. [VSports手机版 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rieser E, Cordier SM, Walczak H. Linear ubiquitination: a newly discovered regulator of cell signalling. Trends Biochem Sci. 2013;38(2):94–102. doi: 10.1016/j.tibs.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Walczak H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev. 2011;244(1):9–28. doi: 10.1111/j.1600-065X.2011.01066.x. ["V体育ios版" DOI] [PubMed] [Google Scholar]
- 7.Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans. 2009;37(Pt 5):937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- 8.Dikic I, Wakatsuki S, Walters KJ. Ubiquitin-binding domains—from structures to functions. Nat Rev Mol Cell Biol. 2009;10(10):659–671. doi: 10.1038/nrm2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10(8):550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 10.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [V体育安卓版 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–227. doi: 10.1016/j.cell.2011.11.031. ["V体育官网入口" DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 15.Harper N, Hughes M, MacFarlane M, Cohen GM. Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor 1 signaling complex during tumor necrosis factor-induced apoptosis. J Biol Chem. 2003;278(28):25534–25541. doi: 10.1074/jbc.M303399200. [DOI] [PubMed] [Google Scholar]
- 16.Vince JE, Pantaki D, Feltham R, Mace PD, Cordier SM, Schmukle AC, Davidson AJ, Callus BA, Wong WW, Gentle IE, Carter H, Lee EF, Walczak H, Day CL, Vaux DL, Silke J. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis. J Biol Chem. 2009;284(51):35906–35915. doi: 10.1074/jbc.M109.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, Feltham R, Vince J, Warnken U, Wenger T, Koschny R, Komander D, Silke J, Walczak H. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36(5):831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Park SM, Yoon JB, Lee TH. Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett. 2004;566(1–3):151–156. doi: 10.1016/j.febslet.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30(6):689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk RG. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA. 2008;105(33):11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283(36):24295–24299. doi: 10.1074/jbc.C800128200. ["V体育官网" DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dynek JN, Goncharov T, Dueber EC, Fedorova AV, Izrael-Tomasevic A, Phu L, Helgason E, Fairbrother WJ, Deshayes K, Kirkpatrick DS, Vucic D. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 2010;29(24):4198–4209. doi: 10.1038/emboj.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, Nachbur U, Gangoda L, Warnken U, Purcell AW, Silke J, Walczak H. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471(7340):591–596. doi: 10.1038/nature09816. ["V体育ios版" DOI] [PubMed] [Google Scholar]
- 24.Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, Tokunaga F, Androulidaki A, Nakagawa T, Pasparakis M, Iwai K, Sundberg JP, Schaefer L, Rittinger K, Macek B, Dikic I. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471(7340):637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, Tanaka K, Nakano H, Iwai K. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471(7340):633–636. doi: 10.1038/nature09815. [VSports最新版本 - DOI] [PubMed] [Google Scholar]
- 26.Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, Yamamoto M, Akira S, Takao T, Tanaka K, Iwai K. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11(2):123–132. doi: 10.1038/ncb1821. ["VSports" DOI] [PubMed] [Google Scholar]
- 27.Smit JJ, Monteferrario D, Noordermeer SM, van Dijk WJ, van der Reijden BA, Sixma TK. The E3 ligase HOIP specifies linear ubiquitin chain assembly through its RING-IBR-RING domain and the unique LDD extension. EMBO J. 2012;31(19):3833–3844. doi: 10.1038/emboj.2012.217. ["V体育官网" DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, Spilgies L, Surinova S, Taraborrelli L, Hartwig T, Rieser E, Martino L, Rittinger K, Walczak H. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015;13(10):2258–2272. doi: 10.1016/j.celrep.2015.11.009. [DOI (V体育2025版)] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412(6844):346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 30.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. TAB 2 and TAB 3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15(4):535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22(2):245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8(4):398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 33.Komander D, Reyes-Turcu F, Licchesi JD, Odenwaelder P, Wilkinson KD, Barford D. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10(5):466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, Kato R, Kensche T, Uejima T, Bloor S, Komander D, Randow F, Wakatsuki S, Dikic I. Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell. 2009;136(6):1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Xu M, Skaug B, Zeng W, Chen ZJ. A ubiquitin replacement strategy in human cells reveals distinct mechanisms of IKK activation by TNFalpha and IL-1beta. Mol Cell. 2009;36(2):302–314. doi: 10.1016/j.molcel.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267(5203):1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 37.Lin YC, Brown K, Siebenlist U. Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alpha: signal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc Natl Acad Sci USA. 1995;92(2):552–556. doi: 10.1073/pnas.92.2.552. [V体育平台登录 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer E, Jiang J, Chen ZJ. Signal-induced ubiquitination of IkappaBalpha by the F-box protein Slimb/beta-TrCP. Genes Dev. 1999;13(3):284–294. doi: 10.1101/gad.13.3.284. [DOI (V体育ios版)] [PMC free article] [PubMed] [Google Scholar]
- 39.Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13(3):270–283. doi: 10.1101/gad.13.3.270. ["V体育官网" DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enesa K, Zakkar M, Chaudhury H, le Luong A, Rawlinson L, Mason JC, Haskard DO, Dean JL, Evans PC. NF-kappaB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. J Biol Chem. 2008;283(11):7036–7045. doi: 10.1074/jbc.M708690200. [DOI] [PubMed] [Google Scholar]
- 41.Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430(7000):694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 42.Ritorto MS, Ewan R, Perez-Oliva AB, Knebel A, Buhrlage SJ, Wightman M, Kelly SM, Wood NT, Virdee S, Gray NS, Morrice NA, Alessi DR, Trost M. Screening of DUB activity and specificity by MALDI-TOF mass spectrometry. Nat Commun. 2014;5:4763. doi: 10.1038/ncomms5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mevissen TE, Hospenthal MK, Geurink PP, Elliott PR, Akutsu M, Arnaudo N, Ekkebus R, Kulathu Y, Wauer T, El Oualid F, Freund SM, Ovaa H, Komander D. OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell. 2013;154(1):169–184. doi: 10.1016/j.cell.2013.05.046. ["V体育2025版" DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De A, Dainichi T, Rathinam CV, Ghosh S. The deubiquitinase activity of A20 is dispensable for NF-kappaB signaling. EMBO Rep. 2014;15(7):775–783. doi: 10.15252/embr.201338305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu TT, Onizawa M, Hammer GE, Turer EE, Yin Q, Damko E, Agelidis A, Shifrin N, Advincula R, Barrera J, Malynn BA, Wu H, Ma A. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity. 2013;38(5):896–905. doi: 10.1016/j.immuni.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu G, Tan X, Wang H, Sun W, Shi Y, Burlingame S, Gu X, Cao G, Zhang T, Qin J, Yang J. Ubiquitin-specific peptidase 21 inhibits tumor necrosis factor alpha-induced nuclear factor kappaB activation via binding to and deubiquitinating receptor-interacting protein 1. J Biol Chem. 2010;285(2):969–978. doi: 10.1074/jbc.M109.042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424(6950):797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 48.Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424(6950):801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 49.Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424(6950):793–796. doi: 10.1038/nature01803. ["VSports" DOI] [PubMed] [Google Scholar]
- 50.Wright A, Reiley WW, Chang M, Jin W, Lee AJ, Zhang M, Sun SC. Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD. Dev Cell. 2007;13(5):705–716. doi: 10.1016/j.devcel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19(10):2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13(19):2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13(12):1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274(5288):787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 57.Rubin BY, Smith LJ, Hellermann GR, Lunn RM, Richardson NK, Anderson SL. Correlation between the anticellular and DNA fragmenting activities of tumor necrosis factor. Cancer Res. 1988;48(21):6006–6010. [PubMed] [Google Scholar]
- 58.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133(4):693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 59.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10(4):348–355. doi: 10.1038/ni.1714. [V体育官网入口 - DOI] [PubMed] [Google Scholar]
- 60.Salvesen GS, Walsh CM. Functions of caspase 8: the identified and the mysterious. Semin Immunol. 2014;26(3):246–252. doi: 10.1016/j.smim.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Donnell MA, Ting AT. NFkappaB and ubiquitination: partners in disarming RIPK1-mediated cell death. Immunol Res. 2012;54(1–3):214–226. doi: 10.1007/s12026-012-8321-7. [DOI] [PubMed] [Google Scholar]
- 62.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12(5):445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moulin M, Anderton H, Voss AK, Thomas T, Wong WW, Bankovacki A, Feltham R, Chau D, Cook WD, Silke J, Vaux DL. IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J. 2012;31(7):1679–1691. doi: 10.1038/emboj.2012.18. [V体育平台登录 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peltzer N, Rieser E, Taraborrelli L, Draber P, Darding M, Pernaute B, Shimizu Y, Sarr A, Draberova H, Montinaro A, Martinez-Barbera JP, Silke J, Rodriguez TA, Walczak H. HOIP deficiency causes embryonic lethality by aberrant TNFR1-mediated endothelial cell death. Cell Rep. 2014;9(1):153–165. doi: 10.1016/j.celrep.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 65.Rickard JA, Anderton H, Etemadi N, Nachbur U, Darding M, Peltzer N, Lalaoui N, Lawlor KE, Vanyai H, Hall C, Bankovacki A, Gangoda L, Wong WW, Corbin J, Huang C, Mocarski ES, Murphy JM, Alexander WS, Voss AK, Vaux DL, Kaiser WJ, Walczak H, Silke J. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. eLife. 2014 doi: 10.7554/eLife.03464. [DOI (VSports)] [PMC free article] [PubMed] [Google Scholar]
- 66.Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, Harris PA, Kaiser WJ, Mocarski ES, Bertin J, Gough PJ. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192(12):5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dondelinger Y, Aguileta MA, Goossens V, Dubuisson C, Grootjans S, Dejardin E, Vandenabeele P, Bertrand MJ. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 2013;20(10):1381–1392. doi: 10.1038/cdd.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gentle IE, Wong WW, Evans JM, Bankovacki A, Cook WD, Khan NR, Nachbur U, Rickard J, Anderton H, Moulin M, Lluis JM, Moujalled DM, Silke J, Vaux DL. In TNF-stimulated cells, RIPK1 promotes cell survival by stabilizing TRAF2 and cIAP1, which limits induction of non-canonical NF-kappaB and activation of caspase-8. J Biol Chem. 2011;286(15):13282–13291. doi: 10.1074/jbc.M110.216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanlangenakker N, Bertrand MJ, Bogaert P, Vandenabeele P, Vanden Berghe T. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2011;2:e230. doi: 10.1038/cddis.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanlangenakker N, Vanden Berghe T, Bogaert P, Laukens B, Zobel K, Deshayes K, Vucic D, Fulda S, Vandenabeele P, Bertrand MJ. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;18(4):656–665. doi: 10.1038/cdd.2010.138. [VSports手机版 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17(5):418–424. doi: 10.1016/j.cub.2007.01.027. ["VSports在线直播" DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43(3):449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43(3):432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 74.HogenEsch H, Gijbels MJ, Offerman E, van Hooft J, van Bekkum DW, Zurcher C. A spontaneous mutation characterized by chronic proliferative dermatitis in C57BL mice. Am J Pathol. 1993;143(3):972–982. [PMC free article] [PubMed] [Google Scholar]
- 75.Seymour RE, Hasham MG, Cox GA, Shultz LD, Hogenesch H, Roopenian DC, Sundberg JP. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007;8(5):416–421. doi: 10.1038/sj.gene.6364403. ["VSports app下载" DOI] [PubMed] [Google Scholar]
- 76.HogenEsch H, Janke S, Boggess D, Sundberg JP. Absence of Peyer’s patches and abnormal lymphoid architecture in chronic proliferative dermatitis (cpdm/cpdm) mice. J Immunol. 1999;162(7):3890–3896. ["VSports手机版" PubMed] [Google Scholar]
- 77.Kumari S, Redouane Y, Lopez-Mosqueda J, Shiraishi R, Romanowska M, Lutzmayer S, Kuiper J, Martinez C, Dikic I, Pasparakis M, Ikeda F. Sharpin prevents skin inflammation by inhibiting TNFR1-induced keratinocyte apoptosis. eLife. 2014 doi: 10.7554/eLife.03422. ["V体育安卓版" DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conze DB, Albert L, Ferrick DA, Goeddel DV, Yeh WC, Mak T, Ashwell JD. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol. 2005;25(8):3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [V体育官网入口 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conte D, Holcik M, Lefebvre CA, Lacasse E, Picketts DJ, Wright KE, Korneluk RG. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol Cell Biol. 2006;26(2):699–708. doi: 10.1128/MCB.26.2.699-708.2006. [VSports注册入口 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157(5):1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci USA. 2014;111(21):7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, Hall C, Spall SK, Phesse TJ, Abud HE, Cengia LH, Corbin J, Mifsud S, Di Rago L, Metcalf D, Ernst M, Dewson G, Roberts AW, Alexander WS, Murphy JM, Ekert PG, Masters SL, Vaux DL, Croker BA, Gerlic M, Silke J. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157(5):1175–1188. doi: 10.1016/j.cell.2014.04.019. ["V体育安卓版" DOI] [PubMed] [Google Scholar]
- 83.Opipari AW, Jr, Hu HM, Yabkowitz R, Dixit VM. The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J Biol Chem. 1992;267(18):12424–12427. [PubMed (V体育官网入口)] [Google Scholar]
- 84.Vereecke L, Sze M, Mc Guire C, Rogiers B, Chu Y, Schmidt-Supprian M, Pasparakis M, Beyaert R, van Loo G. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207(7):1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, Giansanti P, Heck AJ, Dejardin E, Vandenabeele P, Bertrand MJ. NF-kappaB-independent role of IKKalpha/IKKbeta in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell. 2015;60(1):63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 86.Legarda-Addison D, Hase H, O’Donnell MA, Ting AT. NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling. Cell Death Differ. 2009;16(9):1279–1288. doi: 10.1038/cdd.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Donnell MA, Hase H, Legarda D, Ting AT. NEMO inhibits programmed necrosis in an NFkappaB-independent manner by restraining RIP1. PLoS One. 2012;7(7):e41238. doi: 10.1371/journal.pone.0041238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arslan SC, Scheidereit C. The prevalence of TNFalpha-induced necrosis over apoptosis is determined by TAK1–RIP1 interplay. PLoS One. 2011;6(10):e26069. doi: 10.1371/journal.pone.0026069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morioka S, Broglie P, Omori E, Ikeda Y, Takaesu G, Matsumoto K, Ninomiya-Tsuji J. TAK1 kinase switches cell fate from apoptosis to necrosis following TNF stimulation. J Cell Biol. 2014;204(4):607–623. doi: 10.1083/jcb.201305070. [V体育官网入口 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One. 2013;8(10):e76841. doi: 10.1371/journal.pone.0076841. ["V体育安卓版" DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, Prodhomme T, Duong B, Whang MI, Advincula R, Agelidis A, Barrera J, Wu H, Burlingame A, Malynn BA, Zamvil SS, Ma A. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol. 2015;16(6):618–627. doi: 10.1038/ni.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-alpha feedforward signaling. Cell. 2011;145(1):92–103. doi: 10.1016/j.cell.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 93.de Almagro MC, Goncharov T, Newton K, Vucic D. Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination. Cell Death Dis. 2015;6:e1800. doi: 10.1038/cddis.2015.158. ["V体育ios版" DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bertrand MJ, Lippens S, Staes A, Gilbert B, Roelandt R, De Medts J, Gevaert K, Declercq W, Vandenabeele P. cIAP1/2 are direct E3 ligases conjugating diverse types of ubiquitin chains to receptor interacting proteins kinases 1 to 4 (RIP1–4) PLoS One. 2011;6(9):e22356. doi: 10.1371/journal.pone.0022356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petersen SL, Chen TT, Lawrence DA, Marsters SA, Gonzalvez F, Ashkenazi A. TRAF2 is a biologically important necroptosis suppressor. Cell Death Differ. 2015;22(11):1846–1857. doi: 10.1038/cdd.2015.35. [V体育平台登录 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic acids research. 2012;40(database issue):D261–D270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang XD, Sun SC. Targeting signaling factors for degradation, an emerging mechanism for TRAF functions. Immunol Rev. 2015;266(1):56–71. doi: 10.1111/imr.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kulathu Y, Garcia FJ, Mevissen TE, Busch M, Arnaudo N, Carroll KS, Barford D, Komander D. Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nat Commun. 2013;4:1569. doi: 10.1038/ncomms2567. ["VSports app下载" DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajsbaum R, Albrecht RA, Wang MK, Maharaj NP, Versteeg GA, Nistal-Villan E, Garcia-Sastre A, Gack MU. Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein. PLoS Pathog. 2012;8(11):e1003059. doi: 10.1371/journal.ppat.1003059. ["VSports在线直播" DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hartmann T, Xu X, Kronast M, Muehlich S, Meyer K, Zimmermann W, Hurwitz J, Pan ZQ, Engelhardt S, Sarikas A. Inhibition of Cullin-RING E3 ubiquitin ligase 7 by simian virus 40 large T antigen. Proc Natl Acad Sci USA. 2014;111(9):3371–3376. doi: 10.1073/pnas.1401556111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341. [V体育官网 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11(3):290–297. doi: 10.1016/j.chom.2012.01.016. ["V体育平台登录" DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA. 2011;108(50):20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]