IL-15 Enables Septic Shock by Maintaining NK Cell Integrity and Function
- PMID: 28031340
- PMCID: PMC5263185 (VSports)
- DOI: 10.4049/jimmunol.1601486
IL-15 Enables Septic Shock by Maintaining NK Cell Integrity and Function
Abstract
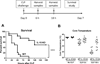
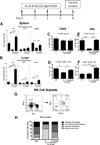
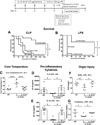
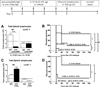
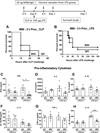
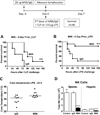
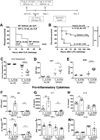
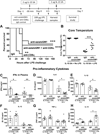
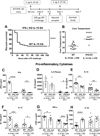
Interleukin 15 is essential for the development and differentiation of NK and memory CD8+ (mCD8+) T cells. Our laboratory previously showed that NK and CD8+ T lymphocytes facilitate the pathobiology of septic shock. However, factors that regulate NK and CD8+ T lymphocyte functions during sepsis are not well characterized. We hypothesized that IL-15 promotes the pathogenesis of sepsis by maintaining NK and mCD8+ T cell integrity. To test our hypothesis, the pathogenesis of sepsis was assessed in IL-15-deficient (IL-15 knockout, KO) mice. IL-15 KO mice showed improved survival, attenuated hypothermia, and less proinflammatory cytokine production during septic shock caused by cecal ligation and puncture or endotoxin-induced shock VSports手机版. Treatment with IL-15 superagonist (IL-15 SA, IL-15/IL-15Rα complex) regenerated NK and mCD8+ T cells and re-established mortality of IL-15 KO mice during septic shock. Preventing NK cell regeneration attenuated the restoration of mortality caused by IL-15 SA. If given immediately prior to septic challenge, IL-15-neutralizing IgG M96 failed to protect against septic shock. However, M96 caused NK cell depletion if given 4 d prior to septic challenge and conferred protection. IL-15 SA treatment amplified endotoxin shock, which was prevented by NK cell or IFN-γ depletion. IL-15 SA treatment also exacerbated septic shock caused by cecal ligation and puncture when given after the onset of sepsis. In conclusion, endogenous IL-15 does not directly augment the pathogenesis of sepsis but enables the development of septic shock by maintaining NK cell numbers and integrity. Exogenous IL-15 exacerbates the severity of sepsis by activating NK cells and facilitating IFN-γ production. .
Copyright © 2017 by The American Association of Immunologists, Inc V体育安卓版. .
Figures




References
-
- Puzanov IJ, Bennett M, Kumar V. IL-15 can substitute for the marrow microenvironment in the differentiation of natural killer cells. Journal of immunology. 1996;157:4282–4285. - PubMed
-
- Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. The Journal of experimental medicine. 2008;205:1213–1225. - PMC (VSports注册入口) - PubMed
-
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. - "VSports手机版" PubMed
-
- Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. Journal of immunology. 2002;168:4827–4831. - PubMed
Publication types
- Actions (V体育2025版)
MeSH terms
- VSports手机版 - Actions
- V体育2025版 - Actions
- VSports app下载 - Actions
- "V体育官网" Actions
- "V体育ios版" Actions
- VSports注册入口 - Actions
Substances
- "V体育官网" Actions
- VSports注册入口 - Actions
V体育官网入口 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育2025版)
Molecular Biology Databases
Research Materials

