Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice
- PMID: 27574188
- PMCID: PMC5147023
- DOI: 10.1182/blood-2016-04-710632
Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice
"V体育官网" Abstract
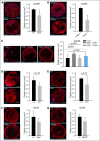
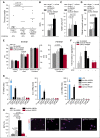
Deep venous thrombosis (DVT) is one of the most common cardiovascular diseases, but its pathophysiology remains incompletely understood. Although sterile inflammation has recently been shown to boost coagulation during DVT, the underlying molecular mechanisms are not fully resolved, which could potentially identify new anti-inflammatory approaches to prophylaxis and therapy of DVT VSports手机版. Using a mouse model of venous thrombosis induced by flow reduction in the vena cava inferior, we identified blood-derived high-mobility group box 1 protein (HMGB1), a prototypical mediator of sterile inflammation, to be a master regulator of the prothrombotic cascade involving platelets and myeloid leukocytes fostering occlusive DVT formation. Transfer of platelets into Hmgb1-/- chimeras showed that this cell type is the major source of HMGB1, exposing reduced HMGB1 on their surface upon activation thereby enhancing the recruitment of monocytes. Activated leukocytes in turn support oxidation of HMGB1 unleashing its prothrombotic activity and promoting platelet aggregation. This potentiates the amount of HMGB1 and further nurtures the accumulation and activation of monocytes through receptor for advanced glycation end products (RAGE) and Toll-like receptor 2, leading to local delivery of monocyte-derived tissue factor and cytokines. Moreover, disulfide HMGB1 facilitates formation of prothrombotic neutrophil extracellular traps (NETs) mediated by RAGE, exposing additional HMGB1 on their extracellular DNA strands. Eventually, a vicious circle of coagulation and inflammation is set in motion leading to obstructive DVT formation. Therefore, platelet-derived disulfide HMGB1 is a central mediator of the sterile inflammatory process in venous thrombosis and could be an attractive target for an anti-inflammatory approach for DVT prophylaxis. .
© 2016 by The American Society of Hematology. V体育安卓版.
Figures
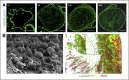
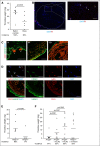
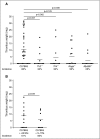

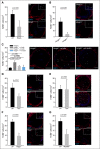
Comment in
-
Platelet HMGB1: the venous clot coordinator.Blood. 2016 Nov 17;128(20):2376-2378. doi: 10.1182/blood-2016-09-738740. Blood. 2016. PMID: 27856468 No abstract available.
References
-
- Brill A, Fuchs TA, Savchenko AS, et al. . Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10(1):136-144. - "VSports最新版本" PMC - PubMed
-
- Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(1):34-45. - PubMed
-
- Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122(7):2331-2336. - VSports手机版 - PMC - PubMed
Publication types
- "V体育平台登录" Actions
MeSH terms
- "V体育平台登录" Actions
- "V体育平台登录" Actions
- "V体育官网" Actions
- VSports - Actions
- Actions (VSports)
- Actions (VSports注册入口)
- VSports注册入口 - Actions
- "V体育2025版" Actions
- VSports在线直播 - Actions
- Actions (VSports手机版)
- "V体育平台登录" Actions
- V体育安卓版 - Actions
Substances (VSports注册入口)
- Actions (VSports注册入口)
- Actions (V体育ios版)
- VSports在线直播 - Actions
- "V体育安卓版" Actions
V体育官网入口 - LinkOut - more resources
"V体育2025版" Full Text Sources
Other Literature Sources
Medical (VSports在线直播)
Molecular Biology Databases

