Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock
- PMID: 26572062
- PMCID: PMC4795157 (VSports在线直播)
- DOI: "V体育官网入口" 10.1016/j.immuni.2015.10.009
Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock
Abstract
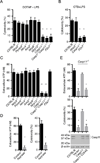
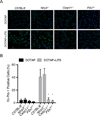
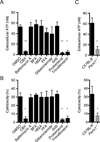
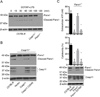
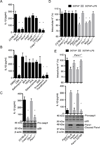
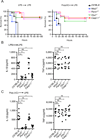
The noncanonical inflammasome induced by intracellular lipopolysaccharide (LPS) leads to caspase-11-dependent pyroptosis, which is critical for induction of endotoxic shock in mice. However, the signaling pathway downstream of caspase-11 is unknown. We found that cytosolic LPS stimulation induced caspase-11-dependent cleavage of the pannexin-1 channel followed up by ATP release, which in turn activated the purinergic P2X7 receptor to mediate cytotoxicity. In the absence of P2X7 or pannexin-1, pyroptosis induced by cytosolic LPS was abrogated. Cleavage of pannexin-1 required the catalytic activity of caspase-11 and was essential for ATP release and P2X7-mediated pyroptosis VSports手机版. Priming the caspase-11 pathway in vivo with LPS or Toll-like receptor-3 (TLR3) agonist resulted in high mortality in wild-type mice after secondary LPS challenge, but not in Casp11(-/-), Panx1(-/-), or P2x7(-/-) mice. These results reveal a critical role for pannexin-1 and P2X7 downstream of caspase-11 for pyroptosis and susceptibility to sepsis induced by the noncanonical inflammasome. .
Copyright © 2015 Elsevier Inc. All rights reserved. V体育安卓版.
Figures

Comment in
-
Pyroptosis: Caspase-11 Unlocks the Gates of Death.Immunity. 2015 Nov 17;43(5):835-7. doi: 10.1016/j.immuni.2015.10.024. Immunity. 2015. PMID: 26588774
References
-
- Bartllet R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014;66(3):638–675. - PubMed
-
- Broz P, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. - PMC (V体育ios版) - PubMed
"V体育安卓版" Publication types
- VSports app下载 - Actions
- V体育ios版 - Actions
MeSH terms
- Actions (VSports最新版本)
- VSports - Actions
- "VSports app下载" Actions
- Actions (VSports在线直播)
- Actions (V体育平台登录)
- "V体育安卓版" Actions
- "V体育ios版" Actions
- Actions (V体育安卓版)
- Actions (V体育官网)
- "VSports注册入口" Actions
- Actions (V体育安卓版)
Substances
- "VSports" Actions
- VSports - Actions
- VSports最新版本 - Actions
- "V体育平台登录" Actions
- "V体育平台登录" Actions
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

