Loop recognition and copper-mediated disulfide reduction underpin metal site assembly of CuA in human cytochrome oxidase
- PMID: 26351686
- PMCID: PMC4586878
- DOI: 10.1073/pnas.1505056112
Loop recognition and copper-mediated disulfide reduction underpin metal site assembly of CuA in human cytochrome oxidase
VSports app下载 - Abstract
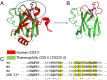
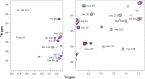
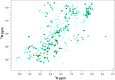
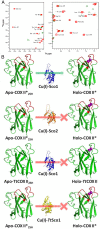
Maturation of cytochrome oxidases is a complex process requiring assembly of several subunits and adequate uptake of the metal cofactors VSports手机版. Two orthologous Sco proteins (Sco1 and Sco2) are essential for the correct assembly of the dicopper CuA site in the human oxidase, but their function is not fully understood. Here, we report an in vitro biochemical study that shows that Sco1 is a metallochaperone that selectively transfers Cu(I) ions based on loop recognition, whereas Sco2 is a copper-dependent thiol reductase of the cysteine ligands in the oxidase. Copper binding to Sco2 is essential to elicit its redox function and as a guardian of the reduced state of its own cysteine residues in the oxidizing environment of the mitochondrial intermembrane space (IMS). These results provide a detailed molecular mechanism for CuA assembly, suggesting that copper and redox homeostasis are intimately linked in the mitochondrion. .
Keywords: CuA site; Sco proteins; cytochrome oxidase; metal site assembly; metallochaperones V体育安卓版. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures


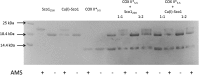
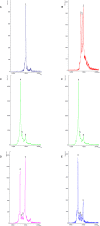
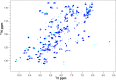
References
-
- Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science. 1999;284(5415):805–808. - PubMed
-
- O’Halloran TV, Culotta VC. Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem. 2000;275(33):25057–25060. - PubMed
-
- Banci L, et al. The Atx1-Ccc2 complex is a metal-mediated protein-protein interaction. Nat Chem Biol. 2006;2(7):367–368. - PubMed (VSports)
"VSports手机版" Publication types
- "V体育安卓版" Actions
MeSH terms
- Actions (V体育官网入口)
- "V体育安卓版" Actions
- "VSports最新版本" Actions
- VSports最新版本 - Actions
- "VSports注册入口" Actions
- V体育安卓版 - Actions
- "V体育官网" Actions
- VSports手机版 - Actions
- V体育官网 - Actions
- "V体育2025版" Actions
- "V体育2025版" Actions
- VSports手机版 - Actions
- "VSports" Actions
Substances
- VSports注册入口 - Actions
- "V体育安卓版" Actions
- "VSports最新版本" Actions
- "VSports最新版本" Actions
- Actions (VSports app下载)
- Actions (VSports最新版本)
LinkOut - more resources
VSports最新版本 - Full Text Sources
Other Literature Sources

