Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation
- PMID: 25673640
- PMCID: PMC5457133 (VSports在线直播)
- DOI: 10.1182/blood-2014-07-590232
Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation
"VSports app下载" Abstract
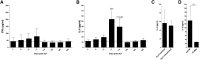
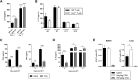
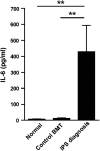
Idiopathic pneumonia syndrome (IPS) is a relatively common, frequently fatal clinical entity, characterized by noninfectious acute lung inflammation following allogeneic stem cell transplantation (SCT), the mechanisms of which are unclear. In this study, we demonstrate that immune suppression with cyclosporin after SCT limits T-helper cell (Th) 1 differentiation and interferon-γ secretion by donor T cells, which is critical for inhibiting interleukin (IL)-6 generation from lung parenchyma during an alloimmune response. Thereafter, local IL-6 secretion induces donor alloantigen-specific Th17 cells to preferentially expand within the lung, and blockade of IL-17A or transplantation of grafts lacking the IL-17 receptor prevents disease. Studies using IL-6(-/-) recipients or IL-6 blockade demonstrate that IL-6 is the critical driver of donor Th17 differentiation within the lung. Importantly, IL-6 is also dysregulated in patients undergoing clinical SCT and is present at very high levels in the plasma of patients with IPS compared with SCT recipients without complications. Furthermore, at the time of diagnosis, plasma IL-6 levels were higher in a subset of IPS patients who were nonresponsive to steroids and anti-tumor necrosis factor therapy. In sum, pulmonary-derived IL-6 promotes IPS via the induction of Th17 differentiation, and strategies that target these cytokines represent logical therapeutic approaches for IPS. VSports手机版.
© 2015 by The American Society of Hematology V体育安卓版. .
V体育官网入口 - Figures




V体育平台登录 - Comment in
-
"VSports" The primacy of IL-6 in IPS?Blood. 2015 Apr 9;125(15):2320-2. doi: 10.1182/blood-2015-02-629816. Blood. 2015. PMID: 25858891
References
-
- Clark JG, Hansen JA, Hertz MI, Parkman R, Jensen L, Peavy HH. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis. 1993;147(6 pt 1):1601–1606. - VSports在线直播 - PubMed
-
- Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. American Thoracic Society Committee on Idiopathic Pneumonia Syndrome. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183(9):1262–1279. - "V体育官网入口" PMC - PubMed
-
- Yanik G, Cooke KR. The lung as a target organ of graft-versus-host disease. Semin Hematol. 2006;43(1):42–52. - PubMed
-
- Burman AC, Banovic T, Kuns RD, et al. IFNgamma differentially controls the development of idiopathic pneumonia syndrome and GVHD of the gastrointestinal tract. Blood. 2007;110(3):1064–1072. - PubMed
"VSports最新版本" Publication types
MeSH terms
- V体育官网入口 - Actions
- Actions (VSports)
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- Actions (V体育官网入口)
- VSports在线直播 - Actions
- V体育官网入口 - Actions
- "VSports" Actions
- "VSports最新版本" Actions
- Actions (VSports注册入口)
- "V体育平台登录" Actions
Substances
- Actions (V体育安卓版)
- Actions (V体育平台登录)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

