V体育官网 - Inhibition of mTOR signaling reduces PELP1-mediated tumor growth and therapy resistance
- PMID: 24688046
- PMCID: PMC4226651
- DOI: 10.1158/1535-7163.MCT-13-0877
Inhibition of mTOR signaling reduces PELP1-mediated tumor growth and therapy resistance
Abstract
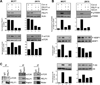
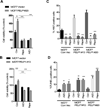
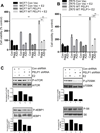
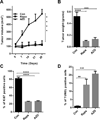
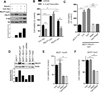
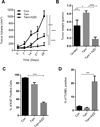
Proline, Glutamic acid-, and Leucine-rich Protein 1 (PELP1) is a proto-oncogene that modulates estrogen receptor (ER) signaling. PELP1 expression is upregulated in breast cancer, contributes to therapy resistance, and is a prognostic marker of poor survival. In a subset of breast tumors, PELP1 is predominantly localized in the cytoplasm and PELP1 participates in extranuclear signaling by facilitating ER interactions with Src and phosphoinositide 3-kinase (PI3K). However, the mechanism by which PELP1 extranuclear actions contributes to cancer progression and therapy resistance remains unclear. In this study, we discovered that PELP1 cross-talked with the serine/threonine protein kinase mTOR and modulated mTOR signaling VSports手机版. PELP1 knockdown significantly reduced the activation of mTOR downstream signaling components. Conversely, PELP1 overexpression excessively activated mTOR signaling components. We detected the presence of the mTOR signaling complex proteins in PELP1 immunoprecipitates. mTOR-targeting drugs (rapamycin and AZD8055) significantly reduced proliferation of PELP1-overexpressed breast cancer cells in both in vitro and in vivo xenograft tumor models. MCF7 cells that uniquely retain PELP1 in the cytoplasm showed resistance to hormonal therapy and mTOR inhibitors sensitized PELP1cyto cells to hormonal therapy in xenograft assays. Notably, immunohistochemical studies using xenograft tumors derived from PELP1 overexpression model cells showed increased mTOR signaling and inhibition of mTOR rendered PELP1-driven tumors to be highly sensitive to therapeutic inhibition. Collectively, our data identified the PELP1-mTOR axis as a novel component of PELP1 oncogenic functions and suggest that mTOR inhibitor(s) will be effective chemotherapeutic agents for downregulating PELP1 oncogenic functions. .
©2014 American Association for Cancer Research V体育安卓版. .
Conflict of interest statement (VSports手机版)
Figures
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Osborne CK, Zhao H, Fuqua SA. Selective estrogen receptor modulators: structure, function, and clinical use. J Clin Oncol. 2000;18:3172–3186. - "V体育ios版" PubMed
-
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–2606. - "V体育官网入口" PubMed
-
- Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101–112. - PubMed
Publication types
MeSH terms
- "V体育官网入口" Actions
- "V体育2025版" Actions
- Actions (V体育官网入口)
- Actions (VSports app下载)
- Actions (V体育官网)
- "VSports注册入口" Actions
- Actions (VSports在线直播)
- VSports - Actions
- V体育安卓版 - Actions
- V体育平台登录 - Actions
- Actions (V体育2025版)
- Actions (VSports最新版本)
- "V体育平台登录" Actions
- "VSports最新版本" Actions
Substances
- Actions (V体育平台登录)
- Actions (VSports手机版)
- V体育官网 - Actions
Grants and funding
V体育官网入口 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
VSports在线直播 - Miscellaneous

