IFN-γ and TNF-α-induced GBP-1 inhibits epithelial cell proliferation through suppression of β-catenin/TCF signaling
- PMID: 22692453
- PMCID: VSports最新版本 - PMC3481006
- DOI: V体育ios版 - 10.1038/mi.2012.41
IFN-γ and TNF-α-induced GBP-1 inhibits epithelial cell proliferation through suppression of β-catenin/TCF signaling
Abstract
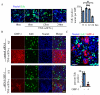
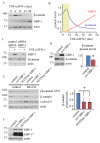
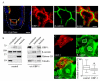
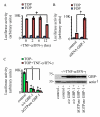
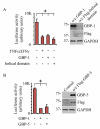
Proinflammatory cytokines induce guanylate-binding protein 1 (GBP-1) protein expression in intestinal epithelial tissues. GBP-1 has been described as influencing a number of cellular processes important for epithelial homeostasis, including cell proliferation VSports手机版. However, many questions remain as to the role of GBP-1 in intestinal mucosal homeostasis. We therefore sought to investigate the function of proinflammatory cytokine-induced GBP-1 during intestinal epithelial cell proliferation. Through the use of complementary GBP-1 overexpression and small interfering RNA-mediated knockdown studies, we now show that GBP-1 acts to inhibit pro-mitogenic β-catenin/T cell factor (TCF) signaling. Interestingly, proinflammatory cytokine-induced GBP-1 was found to be a potent suppressor of β-catenin protein levels and β-catenin serine 552 phosphorylation. Neither glycogen synthase kinase 3β nor proteasomal inhibition alleviated GBP-1-mediated suppression of cell proliferation or β-catenin/TCF signaling, indicating a non-canonical mechanism of β-catenin inhibition. Together, these data show that cytokine-induced GBP-1 retards cell proliferation by forming a negative feedback loop that suppresses β-catenin/TCF signaling. .
Figures

References
-
- Cheng YS, Colonno RJ, Yin FH. Interferon induction of fibroblast proteins with guanylatebinding activity. J Biol Chem. 1983;258:7746–50. - PubMed
-
- Kim BH, et al. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–21. - PubMed
-
- Itsui Y, et al. Antiviral effects of the interferon-induced protein guanylate binding protein 1 and its interaction with the hepatitis C virus NS5B protein. Hepatology. 2009;50:1727–37. - PubMed
-
- Anderson SL, Carton JM, Lou J, Xing L, Rubin BY. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology. 1999;256:8–14. - PubMed (VSports在线直播)
Publication types
- "V体育2025版" Actions
- VSports在线直播 - Actions
"VSports手机版" MeSH terms
- "VSports最新版本" Actions
- Actions (VSports在线直播)
- "V体育平台登录" Actions
- Actions (V体育官网入口)
- VSports - Actions
- VSports手机版 - Actions
- Actions (VSports注册入口)
- "V体育官网" Actions
- "V体育官网入口" Actions
- Actions (V体育安卓版)
- "V体育平台登录" Actions
- VSports - Actions
- Actions (V体育ios版)
- VSports app下载 - Actions
- V体育官网 - Actions
- "VSports在线直播" Actions
Substances
- V体育官网 - Actions
- Actions (VSports app下载)
- V体育安卓版 - Actions
- Actions (VSports)
- Actions (VSports最新版本)
- VSports手机版 - Actions
- "V体育官网入口" Actions
- "VSports注册入口" Actions
- "VSports手机版" Actions
- Actions (V体育官网入口)
Grants and funding
LinkOut - more resources
Full Text Sources
V体育官网入口 - Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

