Deoxycholic acid activates beta-catenin signaling pathway and increases colon cell cancer growth and invasiveness
- PMID: 15004225
- PMCID: PMC404012
- DOI: 10.1091/mbc.e03-12-0894
Deoxycholic acid activates beta-catenin signaling pathway and increases colon cell cancer growth and invasiveness
Abstract
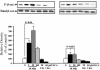
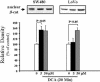
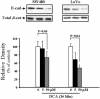
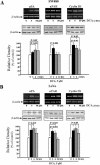
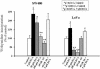
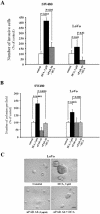
Colorectal cancer is often lethal when invasion and/or metastasis occur. Tumor progression to the metastatic phenotype is mainly dependent on tumor cell invasiveness. Secondary bile acids, particularly deoxycholic acid (DCA), are implicated in promoting colon cancer growth and progression. Whether DCA modulates beta-catenin and promotes colon cancer cell growth and invasiveness remains unknown. Because beta-catenin and its target genes urokinase-type plasminogen activator receptor (uPAR) and cyclin D1 are overexpressed in colon cancers, and are linked to cancer growth, invasion, and metastasis, we investigated whether DCA activates beta-catenin signaling and promotes colon cancer cell growth and invasiveness. Our results show that low concentrations of DCA (5 and 50 microM) significantly increase tyrosine phosphorylation of beta-catenin, induce urokinase-type plasminogen activator, uPAR, and cyclin D1 expression and enhance colon cancer cell proliferation and invasiveness. These events are associated with a substantial loss of E-cadherin binding to beta-catenin. Inhibition of beta-catenin with small interfering RNA significantly reduced DCA-induced uPAR and cyclin D1 expression. Blocking uPAR with a neutralizing antibody significantly suppressed DCA-induced colon cancer cell proliferation and invasiveness. These findings provide evidence for a novel mechanism underlying the oncogenic effects of secondary bile acids. VSports手机版.
"VSports app下载" Figures

"V体育ios版" References
-
- Andreasen, P.A., Kjoller, L., Christensen, L., and Duffy, M.J. (1997). The urokinase-type plasminogen activator system in cancer metastasis: a review. Int. J. Cancer 72, 1–22. - PubMed
-
- Andrews, N.C., and Faller, D.V. (1991). A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acid Res. 19, 2499. - "V体育ios版" PMC - PubMed
-
- Arber, N., Doki, Y., Han, E.K.-H., Sgambato, A., Zhou, P., Kim, N.-H., Delohery, T., Klein, M.G., Holt, P.R., and Weinstein, I.B. (1997). Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res. 57, 1569–1574. - PubMed
-
- Arber, N., Hibshoosh, H., Moss, S.F., Sutter, T., Zhang, Y., Begg, M., Wang, S., Weinstein, I.B., and Holt, P.R. (1996). Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology 110, 669–674. - PubMed (VSports在线直播)
Publication types
- VSports最新版本 - Actions
MeSH terms
- "VSports手机版" Actions
- "VSports" Actions
- VSports - Actions
- "VSports" Actions
- Actions (V体育ios版)
- "V体育安卓版" Actions
- VSports - Actions
- "VSports注册入口" Actions
- "V体育2025版" Actions
- Actions (VSports在线直播)
- V体育官网 - Actions
- Actions (V体育平台登录)
Substances
- "VSports在线直播" Actions
- Actions (VSports最新版本)
- Actions (V体育官网入口)
- Actions (VSports app下载)
- Actions (V体育官网入口)
- Actions (VSports在线直播)
- V体育2025版 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports在线直播)
"VSports最新版本" Research Materials
V体育安卓版 - Miscellaneous

