Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha (VSports app下载)
- PMID: 10713156
- PMCID: PMC85397 (V体育2025版)
- DOI: 10.1128/MCB.20.7.2326-2333.2000
Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha (VSports)
Abstract
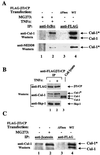
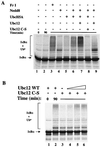
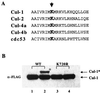
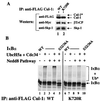
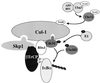
Regulation of NF-kappaB occurs through phosphorylation-dependent ubiquitination of IkappaBalpha, which is degraded by the 26S proteasome. Recent studies have shown that ubiquitination of IkappaBalpha is carried out by a ubiquitin-ligase enzyme complex called SCF(beta(TrCP)). Here we show that Nedd8 modification of the Cul-1 component of SCF(beta(TrCP)) is important for function of SCF(beta(TrCP)) in ubiquitination of IkappaBalpha. In cells, Nedd8-conjugated Cul-1 was complexed with two substrates of SCF(beta(TrCP)), phosphorylated IkappaBalpha and beta-catenin, indicating that Nedd8-Cul-1 conjugates are part of SCF(beta(TrCP)) in vivo VSports手机版. Although only a minute fraction of total cellular Cul-1 is modified by Nedd8, the Cul-1 associated with ectopically expressed betaTrCP was highly enriched for the Nedd8-conjugated form. Moreover, optimal ubiquitination of IkappaBalpha required Nedd8 and the Nedd8-conjugating enzyme, Ubc12. The site of Nedd8 ligation to Cul-1 is essential, as SCF(beta(TrCP)) containing a K720R mutant of Cul-1 only weakly supported IkappaBalpha ubiquitination compared to SCF(beta(TrCP)) containing WT Cul-1, suggesting that the Nedd8 ligation of Cul-1 affects the ubiquitination activity of SCF(beta(TrCP)). These observations provide a functional link between the highly related ubiquitin and Nedd8 pathways of protein modification and show how they operate together to selectively target the signal-dependent degradation of IkappaBalpha. .
Figures

References
-
- Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. - "VSports注册入口" PubMed
-
- Bai C, Sen P, Hofmann K, Ma L, Goebel M, Harper J W, Elledge S J. Skp-1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
-
- Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. - PubMed
Publication types
MeSH terms
- V体育ios版 - Actions
- Actions (VSports)
- "V体育官网入口" Actions
- VSports手机版 - Actions
- "V体育官网" Actions
- V体育2025版 - Actions
- Actions (VSports注册入口)
- "VSports在线直播" Actions
- V体育平台登录 - Actions
"VSports" Substances
- "VSports在线直播" Actions
- Actions (V体育安卓版)
- "VSports手机版" Actions
- Actions (VSports最新版本)
- "VSports app下载" Actions
- VSports最新版本 - Actions
- "V体育官网" Actions
- "V体育安卓版" Actions
- "V体育官网" Actions
- "V体育2025版" Actions
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources (VSports)
VSports最新版本 - Other Literature Sources
Molecular Biology Databases
Miscellaneous
