"VSports手机版" Dysfunctional CD8+ T cells in hepatitis B and C are characterized by a lack of antigen-specific T-bet induction
- PMID: 25225458
- PMCID: PMC4172217
- DOI: 10.1084/jem.20131333
V体育2025版 - Dysfunctional CD8+ T cells in hepatitis B and C are characterized by a lack of antigen-specific T-bet induction
VSports - Abstract
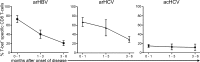
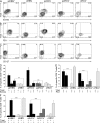
The transcription factor T-bet regulates the production of interferon-γ and cytotoxic molecules in effector CD8 T cells, and its expression correlates with improved control of chronic viral infections. However, the role of T-bet in infections with differential outcome remains poorly defined. Here, we report that high expression of T-bet in virus-specific CD8 T cells during acute hepatitis B virus (HBV) and hepatitis C virus (HCV) infection was associated with spontaneous resolution, whereas T-bet deficiency was more characteristic of chronic evolving infection. T-bet strongly correlated with interferon-γ production and proliferation of virus-specific CD8 T cells, and its induction by antigen and IL-2 stimulation partially restored functionality in previously dysfunctional T-bet-deficient CD8 T cells VSports手机版. However, restoration of a strong interferon-γ response required additional stimulation with IL-12, which selectively induced the phosphorylation of STAT4 in T-bet(+) CD8 T cells. The observation that T-bet expression rendered CD8 T cells responsive to IL-12 suggests a stepwise mechanism of T cell activation in which T-bet facilitates the recruitment of additional transcription factors in the presence of key cytokines. These findings support a critical role of T-bet for viral clearance and suggest T-bet deficiency as an important mechanism behind chronic infection. .
© 2014 Kurktschiev et al.
"VSports最新版本" Figures




Comment in
-
Immunology: viral hepatitis-a critical role for T-bet in viral clearance?Nat Rev Gastroenterol Hepatol. 2014 Nov;11(11):643. doi: 10.1038/nrgastro.2014.168. Epub 2014 Sep 30. Nat Rev Gastroenterol Hepatol. 2014. PMID: 25266112 No abstract available.
References
-
- Artillo, S., Pastore G., Alberti A., Milella M., Santantonio T., Fattovich G., Giustina G., Ryff J.C., Chaneac M., Bartolomé J., and Carreño V.. 1998. Double-blind, randomized controlled trial of interleukin-2 treatment of chronic hepatitis B. J. Med. Virol. 54:167–172 10.1002/(SICI)1096-9071(199803)54:3<167::AID-JMV4>3.0.CO;2-3 - DOI - PubMed
-
- Auffermann-Gretzinger, S., Keeffe E.B., and Levy S.. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 97:3171–3176 10.1182/blood.V97.10.3171 - "VSports app下载" DOI - PubMed
-
- Boni, C., Fisicaro P., Valdatta C., Amadei B., Di Vincenzo P., Giuberti T., Laccabue D., Zerbini A., Cavalli A., Missale G., et al. . 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215–4225 10.1128/JVI.02844-06 - DOI - PMC - PubMed
Publication types
MeSH terms
- "V体育安卓版" Actions
- Actions (V体育ios版)
- "V体育平台登录" Actions
- Actions (VSports手机版)
- VSports手机版 - Actions
- Actions (V体育ios版)
- Actions (VSports最新版本)
- Actions (V体育2025版)
Substances
- VSports手机版 - Actions
- Actions (V体育平台登录)
- "VSports最新版本" Actions
- VSports注册入口 - Actions
Grants and funding
"VSports在线直播" LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports手机版)
Medical (V体育安卓版)
"V体育ios版" Research Materials
Miscellaneous

