Abstract
The objective of this study was to evaluate the effects of combination therapy with photodynamic therapy (PDT) and a novel antiangiogenic regimen using monoclonal antibodies against both vascular endothelial growth factor receptors (VEGFR)-1 (MF1) and VEGFR-2 (DC101) on intracranial glioblastoma xenografts in nude mice. Nude mice bearing intracerebral U87 glioblastoma were treated with PDT and the antiangiogenic regimen (MF1 and DC101) either alone or in combination, while those left untreated served as tumor controls. Tumor volume and animal survival time were analyzed to evaluate the outcome of different treatment modalities. In addition, the immunohistochemical expression of VEGF in the brain adjacent to the tumor, von Willebrand factor (vWF), apoptotic, and proliferative markers in the tumor area were examined VSports最新版本. PDT or MF1 + DC101 alone significantly reduced the tumor volume and prolonged the survival time of glioma-implanted animals. Combined therapy markedly reduced tumor volume and increased survival time with significantly better outcomes than both monotherapies. Both vWF and VEGF levels significantly increased after PDT while they both significantly decreased after antiangiogenic treatment, compared with no treatment. PDT plus anti-angiogenic treatment led to significant decreases in both vWF and VEGF expression, compared with PDT alone. Either PDT or antiangiogenic treatment alone significantly increased tumor cell apoptosis compared with no treatment, while combination therapy resulted in further augmentation of apoptosis. Antiangiogenic treatment with or without PDT significantly decreased tumor cell proliferation, compared with either no treatment or PDT alone. In summary, we demonstrate both significant inhibition of tumor growth and extended survival of mice treated by the combination therapy with PDT and antiangiogenic agents, compared with each single treatment, suggesting that the combination therapy may be a promising strategy to improve clinical outcomes in glioblastoma.
INTRODUCTION
Malignant glioma is both highly vascularized and invasive, characterized by high incidence of recurrence and poor prognosis (1). Angiogenesis is quantitatively most prominent in glioblastoma compared to malignancies elsewhere in the body, and the patterns of growth of invading glioma and angio/vasculogenesis suggest that these processes are fundamentally related V体育平台登录.
Photodynamic therapy (PDT) utilizes a photosensitizer that is more selectively taken up and retained by neoplastic tissue than normal tissue (2-4). When activated by light with an appropriate wavelength, the photosensitizer causes cell death by the production of cytotoxic oxygen products (5). PDT has been extensively investigated, both experimentally and clinically, as an adjunctive treatment of glioblastoma (6). Previously, we have reported that clinically relevant doses of PDT (Photofrin®: 2 mg kg-1; optical: 80 and 120 J cm-2) can significantly shrink the volume of the glioblastoma (7). However, as a side effect, PDT can induce angiogenesis. We found that high-dose PDT (Photofrin®: 12. 5 mg kg-1; optical: 140 J cm-2) induces formation of aberrant new vessels and increases vascular endothelial growth factor (VEGF) levels in normal rat brain (8). Even low-dose PDT (Photofrin®: 2 mg kg-1; optical: 2 and 4 J cm-2) can enhance the expression of VEGF and endothelial cell proliferation in the normal brain of athymic nude mice (9). We also demonstrated that PDT induces expression of VEGF in the brain adjacent to tumor (BAT) in a dose-dependent manner (7). VEGF is an essential angiogenic factor orchestrating glioblastoma angiogenesis (10). Neovascularization and VEGF expression are correlated with the biological aggressiveness, degree of malignancy and clinical recurrence of glioblastoma (11,12). Therefore, the efficacy of PDT in producing tumor regression and cure may be diminished by its pro-angiogenic effects. In contrast, antiangiogenesis may enhance the treatment outcome of PDT VSports注册入口.
Vascular endothelial growth factor and its receptors (VEGFR) are primary angiogenic switches necessary for tumor growth. The combined inhibition of VEGFR-1 and VEGFR-2 signaling is a powerful antiangiogenic treatment, which is more effective than targeting either VEGFR-1 or VEGFR-2 alone in other cancer types (13). However, the combination therapy of PDT with MF1 and DC101 has not yet been studied in experimental glioma models. In this study, we evaluated the effects of combination therapy of PDT with MF1 and DC101 on intracranial glioblastoma xenografts in nude mice V体育官网入口. In addition to evaluating the tumor response, we studied the effects of this combination therapy on the expression pattern of a number of relevant angiogenic factors and on tumor cell proliferation and apoptosis.
MATERIALS AND METHODS (VSports app下载)
All of the experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
V体育平台登录 - U87 glioblastoma cell culture
U87 glioblastoma cells (ATCC, Manassas, VA) were maintained in monolayer culture (37°C, 5% CO2, 95% O2) in minimum essential medium (MEM) with Eagle’s salts supplemented with 10% fetal bovine serum, penicillin, and streptomycin (Gibco, Grand Island, NY). Cells were subcultured and used for implantation when they were in an exponential phase of growth. To harvest, cells were incubated with 0. 05% trypsin EDTA (0. 53 mm, Gibco) for 5 min, and then MEM was added to make a single cell suspension. After the suspension was centrifuged at 200 g (4°C), the medium was removed and the cells were resuspended in PBS. Cell concentration and viability were determined with a cell count using a standard hemacytometer after mixing cells with trypan blue V体育2025版. The suspension was diluted with PBS to a final concentration of 108 cells mL-1.
U87 cell implantation in athymic nude mice
A total of 60 athymic nude mice (20-25 g) obtained from the National Cancer Institute (Frederick, MD), were randomly divided into four groups—tumor control, PDT alone, antiangiogenic treatment (MF1 + DC101) alone and PDT plus antiangiogenic treatment, with 15 animals in each group. The mice were anesthetized with ketamine (80 mg kg-1) and xylazine (13 mg kg-1) administered intraperitoneally (i VSports. p. ). They were placed in a stereotactic apparatus, and after the skull was exposed, a Ø 0. 7 mm burr hole was drilled over the right hemisphere 2. 0 mm lateral to the midline and 1. 0 mm anterior to the bregma. The needle of a 10μL Hamilton syringe was inserted to a depth of 2. 5 mm beneath the dura through the center of the skull hole and 5 × 105 U87 cells in 5 μL PBS were injected intracerebrally during a 5 min interval. The craniectomy was covered with a film of polyvinyl chloride glued to the surrounding intact bone. The incision was closed with 4-0 silk sutures (Ethicon, Somerville, NJ).
Light delivery
A semiconductor diode laser (University Health Network, Toronto, Canada) provided the light (635 ± 5 nm wavelength) for the PDT treatment and optical measurement. The light was coupled into a 200 μm diameter optical fiber with a distal microlens (PDT, Santa Barbara, CA) for a 7 mm diameter, uniform spot for superficial irradiation VSports app下载. The power at the distal end of the fiber was adjusted to 100 mW and was measured before and after each treatment with a power meter (Photodyne, Westlake Village, CA) with a 1 in integrating sphere detector head. The irradiation power was stable in all of the experiments.
PDT treatment
Photodynamic therapy was conducted 7 days after implantation of 5 × 105 U87 cells into the nude mouse brain, when the tumor volume is approximately 1. 1 mm3 according to our previous experience. Photofrin® (Axcan Pharma, Inc. , Birmingham, AL) was dissolved in 5% dextrose solution. Consistent with the clinical dose of Photofrin® (14), 2 mg kg-1 Photofrin® was administered i. p V体育官网. to 30 nude mice on day 6 after tumor implantation. Twenty-four hours after Photofrin® administration, these animals were subjected to laser treatment. The PDT procedure has been previously described in detail (4,8,15). Briefly, animals were anesthetized with ketamine (80 mg kg-1) and xylazine (13 mg kg-1) intramuscularly. After the animals were fixed in a stereotaxic device, a 5-6 mm incision was made directly down the midline, the scalp was retracted and the cranium was exposed. The polyvinyl chloride film, previously glued to the surrounding bone, was removed. Laser light was delivered through a 5 mm craniectomy, with the center located in the small burr hole of the right hemisphere. Based on other reports (14,16) and our experience (7), the optical dose employed in this experiment was 80 J cm-2. The craniectomy was then covered with a new piece of polyvinyl chloride glued to the surrounding intact bone and the incision was closed with 4-0 silk sutures.
Antiangiogenic treatment regimen
Thirty tumor-bearing nude mice, including 15 animals treated with PDT, underwent i.p. injections of monoclonal antibodies (mAbs) against VEGFR-1 (MF1, 400 μg per mouse) and VEGFR-2 (DC101, 800 μg per mouse) every other day from day 8 to day 14 after tumor implantation. The doses of the two agents were based on previous literature (13,17,18) as well as on information obtained from the manufacturer (Imclone, Inc., New York, NY). Animals in both the tumor control (n = 15) and the PDT-alone (n = 15) groups received the same amount of saline i.p. at the same time points.
Survival time measurement
Mean survival time was measured in 10 nude mice from each group. Survival time was determined by either animal death or loss of 20% of body weight.
Histopathology
Five nude mice from each group were killed 21 days after tumor implantation under anesthesia with i.p. administrations of ketamine (80 mg kg-1) and xylazine (13 mg kg-1). The animals were perfused through the left ventricle with 10% neutral buffered formalin following vascular washout with 0.9% saline. Brains were removed and after postfixation they were cut into 1 mm thick blocks which were processed and embedded in paraffin. Six micrometer thick sections were obtained from each of the 1 mm thick blocks and used for histological staining. The slices were stained with hematoxylin and eosin (H&E) for calculation of tumor volume. The tumor volume was measured by an experimenter blinded to the treatment group under a light microscope at 40× magnification. On each coronal section, the area of the tumor was measured by tracing the demarcation of the tumor on the computer screen using the Global Lab Image analysis program (Data Translation, Marlboro, MA), and the tumor volume (mm3) was estimated by the sum of the values produced by multiplying the appropriate tumor area by the section interval thickness.
Immunohistochemistry
A series of 6-μm-thick slices at 50 μm intervals were cut from the block containing the maximum cross-sectional tumor area. von Willebrand factor (vWF), VEGF and Ki67 immunostaining were performed. vWF specifically labels endothelial cells. The Ki67 protein is a nuclear proliferation antigen, which is present in G1,S,G2 and M phases of the cell cycle. Quiescent or resting cells in the G0 phase of the cell cycle do not express the Ki67 antigen. Therefore, determination of Ki67 expression provides a reliable means to define the growth fraction of a population of tumor cells (19,20). A primary rabbit polyclonal IgG antibody against vWF (1:400 dilution; Dako, Carpinteria, CA), a primary goat polyclonal antibody against VEGF (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and a primary rabbit monoclonal antibody against Ki67 (1:300 dilution; Lab Vision Corporation, Fremont, CA) were used, respectively, followed by 3-3′-diaminobenzidine tetrahydrochloride conjugated secondary antibodies (Vector, Burlingame, CA). In situ apoptosis assay was conducted by examining fragmented DNA with the TUNEL ApopTag kit (Chemicon, Temecula, CA) in conjunction with hematoxylin counterstaining. Semi-quantitative measurements of vWF, VEGF, Ki67 and TUNEL immunoreactivity were carried out on five immunostained sections from each brain. Images of corresponding sections were scanned under either a ×40 objective (Olympus BX40, 0.074 mm2 field of view, for VEGF and Ki67) or a ×20 objective (Olympus BX40, 0.296 mm2 field of view, for vWF and TUNEL), using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research, St. Catharines, Canada). For measurement of vascular density and perimeters, three fields from the tumor area in each vWF-immunostained section were visualized (Fig. 1, boxes a-c). The number of vessels was counted and their perimeters were summed up throughout each field of view. For VEGF quantification, four fields from the BAT were selected, and VEGF positive areas in each field of view were measured (Fig. 1, boxes 1-4). VEGF expression is presented as the mean permillage of the positively immunostained area in a field of view. For Ki67 and TUNEL assay, the corresponding positive cells were counted within each of the three fields selected from the tumor area (Fig. 1, boxes a-c). The number of TUNEL-positive cells was divided by the total tissue area to determine cell density (cell number mm-2) and the Ki67 labeling index is the percentage of the number of Ki67-positive cells out of the total number of cells counted in a field of view.
Figure 1.
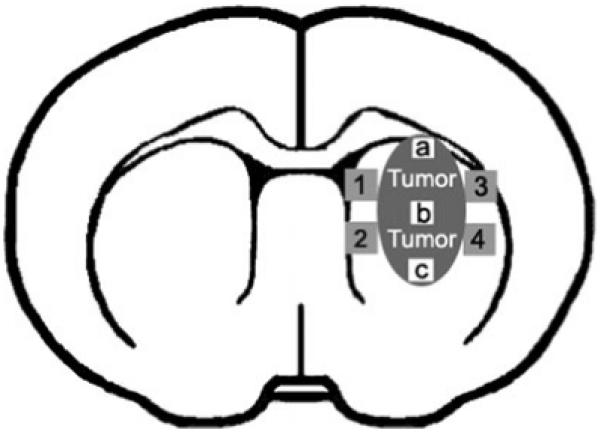
Schematic diagram of a coronal section with four fields selected from the brain adjacent to tumor (1-4) for semi-quantitative measurements of vascular endothelial growth factor expression and three fields selected within the tumor region (a-c) for semi-quantitative measurements of von Willebrand factor, Ki67 and TUNEL immuno-reactivity.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analyses were performed using SPSS software (version 11.5; SPSS, Inc., Chicago, IL). The survival data were analyzed by Kaplan-Meier survival analysis. Statistical comparisons of tumor volume, vessel density and perimeters in the tumor, VEGF expression in the BAT, as well as numbers of apoptotic and proliferative tumor cells between two different treatment groups were made using two-tailed Student’s t-tests. Two-way ANOVA was used to determine whether the two treatment strategies (PDT and antiangiogenic treatment) interact with respect to their effects on survival and tumor volume. P < 0.05 was considered statistically significant.
V体育安卓版 - RESULTS
Tumor volume and survival time
Twenty-one days after tumor implantation, uniform tumor masses were clearly defined by H&E staining observed under a light microscope (Fig. 2). No tumor necrosis was observed in any of the four groups. Measurement of tumor volume revealed that antiangiogenic treatment alone (P < 0.05; Figs. 2b and 3), PDT alone (P < 0.05; Figs. 2c and 3), and PDT plus antiangiogenic treatment (P < 0.01; Figs. 2d and 3) all significantly reduced the tumor volume, compared with no treatment (Figs. 2a and 3). The inhibitive effect of PDT combined with antiangiogenic drugs on glioma growth was in excess of that of either monotherapy (P < 0.05 vs MF1 + DC101; P < 0.05 vs PDT; Figs. 2 and 3). Kaplan-Meier survival analysis showed that either antiangiogenic treatment alone (P < 0.01) or PDT alone (P < 0.01) significantly prolonged the survival time of glioma-bearing mice when compared with the survival time of those without treatment (Fig. 4). Combination therapy with antiangiogenic treatment and PDT significantly increased the survival time beyond that after either monotherapy (P < 0.01 vs MF1 + DC101; P < 0.01 vs PDT; Fig. 4). Two-way ANOVA indicated that there was no significant interaction between the two monotherapy approaches in both tumor volume and survival time. Therefore, the combination of the two therapeutic modalities provided additive but not synergistic treatment effects.
Figure 2.

Representative H&E-stained images under 8× magnification collected at the cross section encompassing the largest tumor area in the brain of untreated tumor-bearing mice (a), mice with glioblastoma receiving MF1 + DC101 treatment (b), mice bearing tumor xenografts treated with photodynamic therapy (PDT) alone (c) and those undergoing antiangiogenic drugs plus PDT (d), killed on day 21 after tumor implantation. Black arrows outline the edge of the tumor mass. Scale bar = 500 μm.
Figure 3.
Measurement of tumor volume. The tumor volume was significantly reduced after treatments by antiangiogenic regimen alone, photodynamic therapy (PDT) alone and combination therapy with antiangiogenic agents and PDT. Antiangiogenic drugs plus PDT further reduced the tumor volume, compared with each single-modality treatment. MF1 (400 μg) and DC101 (800 μg) were administered i.p. every other day from day 8 to day 14 after tumor implantation. PDT (Photofrin®: 2 mg kg-1; optical: 80 J cm-2) was conducted on day 7 after tumor implantation.
Figure 4.

Kaplan-Meier survival plot. MF1 + DC101 treatment (P < 0.01) or photodynamic therapy (PDT) alone (P < 0.01) significantly prolonged the survival time of nude mice bearing U87 glioblastoma compared with no treatment. A significant increase in survival time was observed after combination therapy with PDT and antiangiogenic treatment compared with either monotherapy (P < 0.01 vs MF1 + DC101; P < 0.01 vs PDT). Ten animals in each group were analyzed.
vWF expression in the tumor area
Microvessels with positive vWF immunostaining in the tumor region from different groups are presented in Fig. 5. Semi-quantitative measurements of vWF expression were conducted by calculating the number of vessels and the total perimeter of vessels in the tumor area. Antiangiogenic treatment both with (P < 0.01; Figs. 5d and 6a) and without PDT (P < 0.01; Figs. 5b and 6a) significantly decreased the number of vWF-positive vessels compared with no treatment (Figs. 5a and 6a). However, a significant increase in the number of vWF-positive vessels was found after treatment by PDT alone (Fig. 5c) compared with tumor controls (P < 0.01; Fig. 6a). Combination treatment with PDT and inhibitors of angiogenesis resulted in a significant decrease in the number of vWF-positive vessels compared with PDT alone (P < 0.01; Fig. 6a). Similar results were found in calculation of the total perimeter of microvessels (Fig. 6b). The total perimeter of microvessels was significantly decreased after antiangiogenic treatment either with (P < 0.05) or without PDT (P < 0.01), but significantly increased after PDT alone (P < 0.01), compared with tumor controls. The addition of treatment with the antiangiogenic agents significantly decreased the vessel perimeter, compared with PDT alone (P < 0.01).
Figure 5.

Representative images of von Willebrand factor (vWF) immunohistochemistry. vWF is a marker for endothelial cells. vWF-stained small thin-walled vessels were shown under 200× magnification collected from the tumor area of nude mice bearing glioblastoma with no treatment (a), antiangiogenic treatment alone (b), photodynamic therapy (PDT) alone (c), or antiangiogenic treatment plus PDT (d). Black arrowheads indicate examples of small vessels with positive vWF immunostaining. Bar = 100 μm.
Figure 6.
Semi-quantitative measurement of von Willebrand factor (vWF) expression in the tumor area. Both the number of vWF-positive vessels (a) and the total perimeter of vessels (b) in the tumor area were calculated. MF1 + DC101 with and without photodynamic therapy (PDT) significantly decreased vWF expression compared with no treatment. A significant increase in vWF expression was found after PDT-alone treatment compared with no treatment. PDT combined with antiangiogenic drugs resulted in a significant decrease in vWF immunoreactivity compared with PDT-alone treatment.
VEGF expression in the BAT (V体育平台登录)
Representative images of VEGF immunohistochemistry from different groups are shown in Fig. 7. Semi-quantitative image analysis of VEGF expression revealed significantly increased levels of VEGF in the BAT in glioblastoma-bearing animals subjected to PDT with (P < 0.05; Figs. 7d and 8) or without (P < 0.01; Figs. 7c and 8) antiangiogenic treatment, compared to those without any treatment (Figs. 7a and 8). VEGF immunoreactivity was significantly decreased in mice receiving antiangiogenic drug treatment (Fig. 7b), compared with untreated tumor controls (P < 0.01; Fig. 8). PDT plus antiangiogenic treatment induced a significant decrease in VEGF expression compared with PDT alone (P < 0.01; Fig. 8).
Figure 7.

Representative vascular endothelial growth factor (VEGF) immunostained images under 400× magnification collected from the brain adjacent to tumor of the tumor-bearing brain without any treatment (a), after antiangiogenesis treatment (b), photodynamic therapy (PDT)-alone treatment (c), and antiangiogenic treatment combined with PDT (d), respectively. Black arrowheads indicate examples of sites with positive VEGF labeling. Bar = 50 μm.
Figure 8.
Semi-quantitative measurement of vascular endothelial growth factor (VEGF) expression in the brain adjacent to tumor (BAT). MF1 + DC101 significantly decreased VEGF expression compared with the control group. A significant increase in VEGF expression in the BAT was found after photodynamic therapy (PDT) with or without antiangiogenesis treatment, compared with untreated tumor controls. PDT combined with antiangiogenic drugs significantly decreased VEGF immunoreactivity compared with PDT-alone treatment.
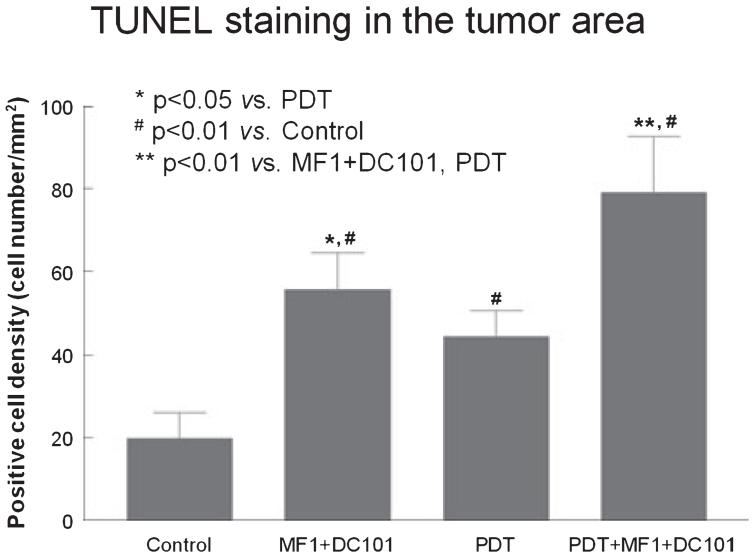
Tumor cell apoptosis
Representative images of TUNEL immunohistochemistry from different groups are shown in Fig. 9. Very few apoptotic cells were found in the tumor of control animals (Fig. 9a). Semi-quantification of TUNEL-positive cells revealed that either antiangiogenic treatment (P < 0.01; Figs. 9b and 10) or PDT (P < 0.01; Figs. 9c and 10) alone significantly increased tumor cell apoptosis, compared to no treatment (Figs. 9a and 10). Furthermore, tumor apoptosis was significantly increased after antiangiogenic treatment combined with PDT (Figs. 9d and 10), when compared with either antiangiogenesis alone (P < 0.01; Figs. 9b and 10) or PDT alone (P < 0.01; Figs. 9c and 10). Antiangiogenic treatment induced a significantly elevated level of apoptosis in tumor cells compared with PDT (P < 0.05; Fig. 10).
Figure 9.

Representative images after TUNEL and hematoxylin counterstaining under 200× magnification, collected from the tumor region of control animals (a), animals with antiangiogenesis treatment (b), those treated with photodynamic therapy (PDT) alone (c) or those subjected to antiangiogenic drugs combined with PDT (d). Arrowheads indicate examples of TUNEL-positive cells. Bar = 100 μm.
Figure 10.
Semi-quantitative measurement of TUNEL immunoreactivity in the tumor area. TUNEL was used to identify apoptotic cells. All of the three treatment strategies resulted in significant increases in tumor cell apoptosis, compared with no treatment. Combination therapy with antiangiogenic agents and photodynamic therapy (PDT) significantly increased the rate of tumor cell apoptosis compared with either antiangiogenic treatment alone or PDT alone.
Ki67 immunoreactivity in the tumor area
Representative images of Ki67 immunohistochemistry from different groups are shown in Fig. 11. There were no significant differences in tumor cell proliferation, as indicated by Ki67 immunoreactivity, between the PDT-alone group (Fig. 11c) and the control group (Fig. 11a). Antiangiogenic treatment with (P < 0.01; Figs. 11d and 12) and without (P < 0.01; Figs. 11b and 12) PDT significantly decreased Ki67 expression in the tumor area, compared with the no treatment group (Figs. 11a and 12). Compared with PDT alone, PDT plus antiangiogenic drug treatment significantly decreased the tumor cell proliferation rate (P < 0.01; Fig. 12).
Figure 11.

Representative Ki67 immunostained images under 400× magnification collected from the tumor region of glioblastoma-bearing mice with no treatment (a), antiangiogenesis treatment alone (b), photodynamic therapy (PDT) alone (c) or antiangiogenic treatment plus PDT (d). Dark brown cells are Ki67-positive cells. Bar = 50 μm.
Figure 12.
Semi-quantitative measurement of Ki67 expression in the tumor region. Ki67 is a robust marker of proliferative cells. Photodynamic therapy (PDT) alone did not affect tumor cell proliferation. MF1 + DC101 with or without PDT resulted in a significant decrease in Ki67 expression in the tumor area compared with either no treatment or PDT alone.
DISCUSSION
In the current study, we demonstrated that antiangiogenic agents combined with PDT significantly reduced tumor volume and prolonged survival time of nude mice bearing glioblastoma, compared with either no treatment or monotherapies.
Angiogenesis is defined as the growth of new blood vessels from preexisting ones. Among the most substantial mediators of angiogenesis are VEGF and its receptors (21), whose physiological importance in blood vessel formation has been clearly demonstrated in gene knockout experiments. Targeted deletion of the VEGF gene (22), VEGFR-2 (Flk-1/KDR) gene (23), and VEGFR-1 (Flt-1) gene (24) in mice resulted in embryonic lethal phenotypes. Angiogenesis also plays a key role in numerous human diseases, including malignant tumor progression (25,26). VEGF is abundantly expressed in a variety of human tumors (27) and its expression is strongly upregulated by hypoxia and oncogenes that are tightly associated with rapidly growing tumors (28,29). VEGFRs have been shown to be involved in the regulation of tumor angiogenesis in various tumors (30). Whereas VEGFR-2 seems to mediate the major growth and permeability actions of VEGF, VEGFR-1 may act either as a decoy receptor or as suppressing signaling through VEGFR-2 (31).
Photodynamic therapy has improved the outcome of central nervous system neoplasms in experimental models in our and other laboratories (4,15,32-35). Clinical studies also suggested that PDT improved survival time in patients with malignant glioma (36,37). PDT offers localized tumoricidal activity based on photosensitizer retention in tumor tissue and the laser-induced photochemical production of reactive oxygen species, leading to tumor necrosis and apoptosis (14,33-35,38). In this study, we found that PDT alone markedly reduced tumor size and extended survival time coincident with increased tumor cell apoptosis, but did not affect tumor cell proliferation. We also demonstrated that PDT induced angiogenesis in both the tumor and the brain tissue around the tumor, as indicated by measurements of vWF and VEGF proteins. The possible relationships between PDT and angiogenesis were first proposed by Ferrario et al. (39), indicating that Photofrin-induced PDT produced significant increases in VEGF within treated lesions by inducing expression of HIF-1a and increasing protein levels of the HIF-1 target gene, VEGF. We considered that in addition to an incomplete tumor cell kill, the ultimate failure of PDT as a treatment of glioma, might be attributed to enhanced angiogenesis powered by PDT induction of VEGF.
Antiangiogenesis therapy is aimed at cutting the blood supply of a tumor, and thus inhibiting or destroying the tumor itself (40). One of the antiangiogenesis therapeutic strategies is to suppress the activity of the major angiogenic regulators, VEGF and its receptors (41), including antisense VEGF (42), anti-VEGF mAb (43), soluble VEGFR (44), Flk-1/kinase insert domain-containing receptor kinase inhibitor (45) and anti-VEGFR mAb (18).
In this study, anti-mouse neutralizing antibodies against both VEGFR-1 (MF1) and VEGFR-2 (DC101) were used, which provide one example of a powerful antiangiogenic treatment, more effective than targeting either VEGFR1 or VEGFR2 alone in other cancer types (13). The results of this study demonstrated that systemic administration of MF1 and DC101 inhibited angiogenesis and growth of human glioblastoma xenografts and extended the survival time of glioma-bearing animals. Furthermore, we observed a decrease in proliferative tumor cells and an increase in apoptotic tumor cells in mice with antiangiogenic treatment. Our data suggest that the inhibition of tumor growth is caused by the blockade of VEGFR signaling, which results in the lack of new vasculature and possibly growth and survival factors produced by the tumor vasculature to supply the rapidly growing tumor mass.
Many studies have been conducted to search for alternative modalities of tumor treatment including radiation therapy and chemotherapy in combination with antiangiogenic therapy. A newly developed strategy combining continuous low-dose chemotherapy and antiangiogenesis has been employed in the treatment of experimental tumors (46-48), which was found to suppress tumor growth more effectively than conventional chemotherapy alone, with a considerably lower occurrence of side effects, even when the tumors underwent drug-resistant conditioning before therapy.
The therapeutic responsiveness of PDT combined with antiangiogenic treatment has been recently evaluated in many cancer types. Kosharskyy et al. (49) reported that subcurative PDT in an orthotopic model of prostate cancer (LNCaP) increased not only VEGF secretion but also the fraction of animals with lymph node metastases. PDT followed by administration of an antiangiogenic agent, TNP-470, abolished this increase and reduced local tumor growth. A VEGF inhibitor, Avastin, enhanced PDT treatment of Kaposi’s sarcoma in a mouse tumor model (50). Antiangiogenic agents (i.e. SU5416 and SU6668) were shown to promote the therapeutic responsiveness of PDT in murine nasopharyngeal carcinoma models (51). However, the combination therapy has not yet been applied in glioma management. Whether or not antiangiogenic agents can counter the upregulated VEGF expression and action induced by PDT and thereby increase the responsiveness of glioma to PDT has not been studied.
Survival is the ultimate “gold standard” endpoint assay for evaluation of tumor treatment response. In this study, although PDT or antiangiogenic therapy alone indeed prolonged the survival time of the animals with glioblastoma, animals treated by the combination therapy had a significantly longer survival time than those receiving a single treatment modality. Concurrently, we found that the tumor response to this combination therapy was greater than each of the single treatments in both direct inhibition of tumor growth and prevention of secondary tumor growth by inhibiting tumor angiogenesis and increasing tumor cell apoptosis. PDT alone may shrink or destroy tumors; however, it induces angiogenesis which promotes tumor regrowth. Antiangiogenic treatment inhibits the growth of tumor cells through cutting off the tumor’s nutritional supply. Our data showed that the combination therapy provided additive anti-cancer efficacy in that the angiogenesis induced by PDT was counteracted by the antiangiogenic agents.
In summary, we have shown that combination therapy with PDT and antiangiogenic agents inhibits glioblastoma angiogenesis, leading to a decrease in tumor volume and an extension in survival time, with better anti-cancer efficacy than PDT or antiangiogenic agents administered alone. Therefore, the combination treatment may be a reasonable strategy to improve clinical outcomes in glioblastoma.
Acknowledgements
This work was supported by National Institutes of Health grants PO1 CA043892 and RO1 CA100486. The authors thank Cynthia Roberts, Sutapa Santra and Qing-e Lu for technical assistance.
REFERENCES (V体育2025版)
- 1.Nicholas MK, Prados MD, Larson DA. Malignant astrocytomas. In: Black PM, Loeffler J, editors. Cancer of the Nervous System. Blackwell Science; Cambridge, MA: 1997. pp. 464–491. [Google Scholar]
- 2.Muller PJ, Wilson BC. Photodynamic therapy of malignant brain tumours. Can. J. Neurol. Sci. 1990;17:193–198. doi: 10.1017/s0317167100030444. [DOI] [PubMed] [Google Scholar]
- 3.Pass HI. Photodynamic therapy in oncology: Mechanisms and clinical use. J. Natl Cancer Inst. 1993;85:443–456. doi: 10.1093/jnci/85.6.443. [DOI] [PubMed] [Google Scholar]
- 4.Jiang F, Lilge L, Grenier J, Li Y, Wilson MD, Chopp M. Photodynamic therapy of U87 human glioma in nude rat using liposome-delivered photofrin. Lasers Surg. Med. 1998;22:74–80. doi: 10.1002/(sici)1096-9101(1998)22:2<74::aid-lsm2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Wilson BC. Photodynamic therapy for cancer: Principles. Can. J. Gastroenterol. 2002;16:393–396. doi: 10.1155/2002/743109. [DOI] [PubMed] [Google Scholar]
- 6.Kostron H, Obwegeser A, Jakober R. Photodynamic therapy in neurosurgery: A review. J. Photochem. Photobiol. B. 1996;36:157–168. doi: 10.1016/s1011-1344(96)07364-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Jiang F, Kalkanis SN, Zhang Z, Hong X, Yang H, Chopp M. Post-acute response of 9L gliosarcoma to Photofrin-mediated PDT in athymic nude mice. Lasers Med. Sci. 2007 doi: 10.1007/s10103-007-0442-1. DOI: 10.1007/s10103-007-0442-1. [DOI] [PubMed] [Google Scholar]
- 8.Jiang F, Zhang ZG, Katakowski M, Robin AM, Faber M, Zhang F, Chopp M. Angiogenesis induced by photodynamic therapy in normal rat brains. Photochem. Photobiol. 2004;79:494–498. doi: 10.1562/2003-11-19-rc.1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Jiang F, Zhang ZG, Kalkanis SN, Hong X, Decarvalho AC, Chen J, Yang H, Robin AM, Chopp M. Low-dose photodynamic therapy increases endothelial cell proliferation and VEGF expression in nude mice brain. Lasers Med. Sci. 2005;20:74–79. doi: 10.1007/s10103-005-0348-8. [DOI] [PubMed] [Google Scholar]
- 10.Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: Coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int. J. Cancer. 1994;59:520–529. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 11.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer. 1996;77:362–372. doi: 10.1002/(SICI)1097-0142(19960115)77:2<362::AID-CNCR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Maia AC, Jr, Malheiros SM, da Rocha AJ, da Silva CJ, Gabbai AA, Ferraz FA, Stavale JN. MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. Am. J. Neuroradiol. 2005;26:777–783. [V体育ios版 - PMC free article] [PubMed] [Google Scholar]
- 13.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 14.Muller PJ, Wilson BC. Photodynamic therapy for malignant newly diagnosed supratentorial gliomas. J. Clin. Laser Med. Surg. 1996;14:263–270. doi: 10.1089/clm.1996.14.263. [DOI] [PubMed] [Google Scholar]
- 15.Jiang F, Lilge L, Logie B, Li Y, Chopp M. Photodynamic therapy of 9L gliosarcoma with liposome-delivered photofrin. Photochem. Photobiol. 1997;65:701–706. doi: 10.1111/j.1751-1097.1997.tb01913.x. ["V体育ios版" DOI] [PubMed] [Google Scholar]
- 16.Muller PJ, Wilson BC. Photodynamic therapy for recurrent supratentorial gliomas. Semin. Surg. Oncol. 1995;11:346–354. doi: 10.1002/ssu.2980110504. [VSports手机版 - DOI] [PubMed] [Google Scholar]
- 17.Kunkel P, Ulbricht U, Bohlen P, Brockmann MA, Fill-brandt R, Stavrou D, Westphal M, Lamszus K. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed (V体育安卓版)] [Google Scholar]
- 18.Prewett M, Huber J, Li Y, Santiago A, O’Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 19.Shiba M, Kohno H, Kakizawa K, Iizasa T, Otsuji M, Saitoh Y, Hiroshima K, Ohwada H, Fujisawa T. Ki-67 immunostaining and other prognostic factors including tobacco smoking in patients with resected nonsmall cell lung carcinoma. Cancer. 2000;89:1457–1465. doi: 10.1002/1097-0142(20001001)89:7<1457::aid-cncr7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 21.Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin. Cancer Biol. 1999;9:211–220. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [V体育官网 - DOI] [PubMed] [Google Scholar]
- 23.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 24.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 25.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. ["V体育官网入口" DOI] [PubMed] [Google Scholar]
- 27.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [V体育平台登录 - PMC free article] [PubMed] [Google Scholar]
- 28.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 29.Rak J, Filmus J, Finkenzeller G, Grugel S, Marme D, Kerbel RS. Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Rev. 1995;14:263–277. doi: 10.1007/BF00690598. [DOI] [PubMed] [Google Scholar]
- 30.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- 31.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 32.Chopp M, Madigan L, Dereski M, Jiang F, Li Y. Photodynamic therapy of human glioma (U87) in the nude rat. Photochem. Photobiol. 1996;64:707–711. doi: 10.1111/j.1751-1097.1996.tb03128.x. [DOI] [PubMed] [Google Scholar]
- 33.Jiang F, Lilge L, Belcuig M, Singh G, Grenier J, Li Y, Chopp M. Photodynamic therapy using Photofrin in combination with buthionine sulfoximine (BSO) to treat 9L gliosarcoma in rat brain. Lasers Surg. Med. 1998;23:161–166. doi: 10.1002/(sici)1096-9101(1998)23:3<161::aid-lsm5>3.0.co;2-n. [VSports注册入口 - DOI] [PubMed] [Google Scholar]
- 34.Lilge L, Portnoy M, Wilson BC. Apoptosis induced in vivo by photodynamic therapy in normal brain and intracranial tumour tissue. Br. J. Cancer. 2000;83:1110–1117. doi: 10.1054/bjoc.2000.1426. [VSports最新版本 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George JE, 3rd, Ahmad Y, Varghai D, Li X, Berlin J, Jackowe D, Jungermann M, Wolfe MS, Lilge L, Totonchi A, Morris RL, Peterson A, Lust WD, Kenney ME, Hoppel CL, Sun J, Oleinick NL, Dean D. Pc 4 photodynamic therapy of U87-derived human glioma in the nude rat. Lasers Surg. Med. 2005;36:383–389. doi: 10.1002/lsm.20185. [DOI] [PubMed] [Google Scholar]
- 36.Kostron H, Plangger C, Fritsch E, Maier H. Photodynamic treatment of malignant brain tumors. Wien. Klin. Wochenschr. 1990;102:531–535. [PubMed (VSports最新版本)] [Google Scholar]
- 37.Muller P, Wilson B. Photodynamic therapy of brain tumours—Post-operative “field fractionation.”. J. Photochem. Photobiol. B. 1991;9:117–119. doi: 10.1016/1011-1344(91)80009-7. ["V体育安卓版" DOI] [PubMed] [Google Scholar]
- 38.Stylli SS, Kaye AH, MacGregor L, Howes M, Rajendra P. Photodynamic therapy of high grade glioma—Long term survival. J. Clin. Neurosci. 2005;12:389–398. doi: 10.1016/j.jocn.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Ferrario A, von Tiehl KF, Rucker N, Schwarz MA, Gill PS, Gomer CJ. Antiangiogenic treatment enhances photodynamic therapy responsiveness in a mouse mammary carcinoma. Cancer Res. 2000;60:4066–4069. [PubMed] [Google Scholar]
- 40.Zhang L, Yu D, Hicklin DJ, Hannay JA, Ellis LM, Pollock RE. Combined anti-fetal liver kinase 1 monoclonal antibody and continuous low-dose doxorubicin inhibits angiogenesis and growth of human soft tissue sarcoma xenografts by induction of endothelial cell apoptosis. Cancer Res. 2002;62:2034–2042. [PubMed] [Google Scholar]
- 41.Hagedorn M, Bikfalvi A. Target molecules for antiangiogenic therapy: From basic research to clinical trials. Crit. Rev. Oncol. Hematol. 2000;34:89–110. doi: 10.1016/s1040-8428(00)00056-1. [DOI (V体育官网入口)] [PubMed] [Google Scholar]
- 42.Im SA, Gomez-Manzano C, Fueyo J, Liu TJ, Ke LD, Kim JS, Lee HY, Steck PA, Kyritsis AP, Yung WK. Antiangiogenesis treatment for gliomas: Transfer of anti-sense-vascular endothelial growth factor inhibits tumor growth in vivo. Cancer Res. 1999;59:895–900. [PubMed (VSports手机版)] [Google Scholar]
- 43.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 44.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl Acad. Sci. USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed (V体育官网入口)] [Google Scholar]
- 46.Kakeji Y, Teicher BA. Preclinical studies of the combination of angiogenic inhibitors with cytotoxic agents. Invest. New Drugs. 1997;15:39–48. doi: 10.1023/a:1005718628223. [DOI] [PubMed] [Google Scholar]
- 47.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J. Clin. Invest. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J. Clin. Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosharskyy B, Solban N, Chang SK, Rizvi I, Chang Y, Hasan T. A mechanism-based combination therapy reduces local tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer Res. 2006;66:10953–10958. doi: 10.1158/0008-5472.CAN-06-1793. [DOI (VSports app下载)] [PubMed] [Google Scholar]
- 50.Ferrario A, Gomer CJ. Avastin enhances photodynamic therapy treatment of Kaposi’s sarcoma in a mouse tumor model. J. Environ. Pathol. Toxicol. Oncol. 2006;25:251–259. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.160. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Q, Olivo M, Lye KY, Moore S, Sharma A, Chowbay B. Enhancing the therapeutic responsiveness of photodynamic therapy with the antiangiogenic agents SU5416 and SU6668 in murine nasopharyngeal carcinoma models. Cancer Chemother. Pharmacol. 2005;56:569–577. doi: 10.1007/s00280-005-1017-0. [DOI] [PubMed] [Google Scholar]