Neutrophil Extracellular Traps Impair Intestinal Barrier Function during Experimental Colitis
- PMID: 32764411
- PMCID: PMC7459452
- DOI: 10.3390/biomedicines8080275
Neutrophil Extracellular Traps Impair Intestinal Barrier Function during Experimental Colitis
Abstract
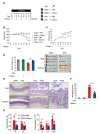
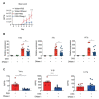
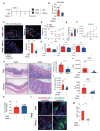
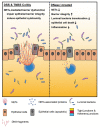
Aberrant neutrophil extracellular trap (NET) formation and the loss of barrier integrity in inflamed intestinal tissues have long been associated with inflammatory bowel disease (IBD). However, whether NETs alter intestinal epithelium permeability during colitis remains elusive. Here, we demonstrated that NETs promote the breakdown in intestinal barrier function for the pathogenesis of intestinal inflammation in mouse models of colitis. NETs were abundant in the colon of mice with colitis experimentally induced by dextran sulfate sodium (DSS) or 2,4,6-trinitrobenzene sulfonic acid (TNBS) VSports手机版. Analysis of the intestinal barrier integrity revealed that NETs impaired gut permeability, enabling the initiation of luminal bacterial translocation and inflammation. Furthermore, NETs induced the apoptosis of epithelial cells and disrupted the integrity of tight junctions and adherens junctions. Intravenous administration of DNase I, an enzyme that dissolves the web-like DNA filaments of NETs, during colitis restored the mucosal barrier integrity which reduced the dissemination of luminal bacteria and attenuated intestinal inflammation in both DSS and TNBS models. We conclude that NETs serve a detrimental factor in the gut epithelial barrier function leading to the pathogenesis of mucosal inflammation during acute colitis. .
Keywords: DNase I; DSS/TNBS-induced colitis; intestinal barrier integrity; neutrophil extracellular traps (NETs) V体育安卓版. .
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results V体育ios版.
Figures



References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
-
- Khor B., Gardet A., Xavier R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. - VSports手机版 - DOI - PMC - PubMed
-
- Knights D., Lassen K.G., Xavier R.J. Advances in inflammatory bowel disease pathogenesis: Linking host genetics and the microbiome. Gut. 2013;62:1505–1510. doi: 10.1136/gutjnl-2012-303954. - DOI (VSports最新版本) - PMC - PubMed
-
- Wera O., Lancellotti P., Oury C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016;5:118. doi: 10.3390/jcm5120118. - DOI (V体育ios版) - PMC - PubMed
VSports注册入口 - Grants and funding
LinkOut - more resources
Full Text Sources

