Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems
- PMID: 31072061
- PMCID: PMC6562917
- DOI: 10.3390/cancers11050640
Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems
Abstract
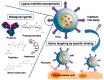
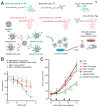
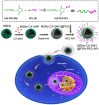
Targeting nanoparticle (NP) carriers to sites of disease is critical for their successful use as drug delivery systems VSports手机版. Along with optimization of physicochemical properties, researchers have focused on surface modification of NPs with biological ligands. Such ligands can bind specific receptors on the surface of target cells. Furthermore, biological ligands can facilitate uptake of modified NPs, which is referred to as 'active targeting' of NPs. In this review, we discuss recent applications of biological ligands including proteins, polysaccharides, aptamers, peptides, and small molecules for NP-mediated drug delivery. We prioritized studies that have demonstrated targeting in animals over in vitro studies. We expect that this review will assist biomedical researchers working with NPs for drug delivery and imaging. .
Keywords: active targeting; biodistribution; drug delivery; ligand; nanoparticle; tumor targeting. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hare J.I., Lammers T., Ashford M.B., Puri S., Storm G., Barry S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. - DOI (V体育安卓版) - PubMed
-
- Aaron C.A., Balabhaskar P., Kapil P., Samir M. Clinical and commercial translation of advanced polymeric nanoparticle systems: Opportunities and material challenges. Transl. Mater. Res. 2017;4 doi: 10.1088/2053-1613/aa5468. - DOI (V体育2025版)
-
- Barenholz Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. - DOI (VSports注册入口) - PubMed
-
- Göke K., Lorenz T., Repanas A., Schneider F., Steiner D., Baumann K., Bunjes H., Dietzel A., Finke J.H., Glasmacher B., Kwade A. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2018;126:40–56. doi: 10.1016/j.ejpb.2017.05.008. - V体育平台登录 - DOI - PubMed
-
- Xie J., Lee S., Chen X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010;62:1064–1079. doi: 10.1016/j.addr.2010.07.009. - DOI (V体育官网入口) - PMC - PubMed
Publication types
- Actions (VSports注册入口)
Grants and funding
LinkOut - more resources
Full Text Sources
"VSports app下载" Other Literature Sources
Miscellaneous

