Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation (V体育平台登录)
- PMID: 9832561
- PMCID: PMC2133072
- DOI: VSports手机版 - 10.1083/jcb.143.5.1341
V体育官网入口 - Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation
"V体育2025版" Abstract
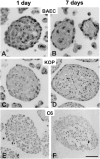
Single endothelial cells (EC) seeded in suspension culture rapidly undergo apoptosis. Addition of survival factors, such as VEGF and FGF-2, does not prevent apoptosis of suspended EC. However, when cells are allowed to establish cell-cell contacts, they become responsive to the activities of survival factors. These observations have led to the development of a three-dimensional spheroid model of EC differentiation. EC spheroids remodel over time to establish a differentiated surface layer of EC and a center of unorganized EC that subsequently undergo apoptosis. Surface EC become quiescent, establish firm cell-cell contacts, and can be induced to express differentiation antigens (e. g. , induction of CD34 expression by VEGF). In contrast, the unorganized center spheroid cells undergo apoptosis if they are not rescued by survival factors. The responsiveness to the survival factor activities of VEGF and FGF-2 was not dependent on cell shape changes since it was retained after cytochalasin D treatment VSports手机版. Taken together, these findings characterize survival factor requirements of unorganized EC and indicate that polarized surface EC differentiate to become independent of exogenous survival factors. Furthermore, they demonstrate that spheroid cell culture systems are useful not just for the study of tumor cells and embryonic stem cells but also for the analysis of differentiated functions of nontransformed cells. .
VSports在线直播 - Figures











References
-
- Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. - PubMed
-
- Arras M, Mollnau H, Strasser R, Wenz R, Ito WD, Schaper J, Schaper W. The delivery of angiogenic factors to the heart by microsphere therapy. Nat Biotechnol. 1998;16:159–162. - VSports - PubMed
-
- Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:II365–II371. - "V体育ios版" PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:965–967. - PubMed (VSports手机版)
-
- Augustin HG, Voss AK, Pauli BU. Senescence of aortic endothelial cells in culture: effects of basic fibroblast growth factor expression on cell phenotype, migration, and proliferation. J Cell Physiol. 1993;157:279–288. - PubMed
Publication types
- Actions (V体育ios版)
MeSH terms
- Actions (V体育平台登录)
- V体育官网入口 - Actions
- V体育官网入口 - Actions
- "VSports app下载" Actions
- Actions (V体育2025版)
- VSports在线直播 - Actions
- V体育ios版 - Actions
- "V体育官网入口" Actions
- Actions (VSports最新版本)
- "V体育官网" Actions
- V体育安卓版 - Actions
- Actions (V体育安卓版)
Substances
- Actions (VSports最新版本)
- "VSports手机版" Actions
- VSports app下载 - Actions
- V体育官网 - Actions
- V体育ios版 - Actions
- VSports最新版本 - Actions
- "VSports最新版本" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

