Addition of oncolytic virotherapy to clinical isolated limb perfusion for melanoma and sarcoma activates antitumor immunity
- PMID: 41005981
- PMCID: PMC12481388
- DOI: 10.1136/jitc-2025-012446
Addition of oncolytic virotherapy to clinical isolated limb perfusion for melanoma and sarcoma activates antitumor immunity
Abstract
Background: We previously showed that oncolytic virotherapy delivered by isolated limb perfusion (ILP), combined with immune checkpoint inhibition, prevents both local tumor progression and systemic metastases in an animal sarcoma model VSports手机版. .
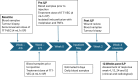
Methods: We describe a first-in-human phase I/II trial combining oncolytic herpes simplex virus, talimogene laherparepvec (T-VEC), with melphalan and tumor necrosis factor-alpha delivered by ILP, in patients with locally advanced sarcoma or melanoma. V体育安卓版.
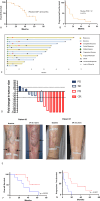
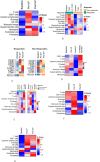
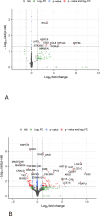
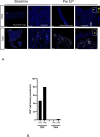
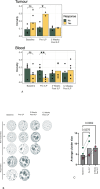
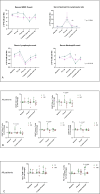
Results: T-VEC/ILP is well tolerated, with an overall response rate of 53% in all patients and 44% in sarcoma. Importantly, we report durable complete responses in sarcoma subtypes usually unresponsive to ILP. Translational analysis of longitudinal tumor and blood samples showed that T-VEC induced an inflammatory gene expression profile within injected tumors, which was more sustained in sarcoma than in melanoma. In relation to clinical outcome, responding patients with sarcoma showed a greater increase in gene expression for interferon response after virus treatment than non-responding patients V体育ios版. Analysis of the T-cell repertoire (TCR) in tumor and blood showed that clonality was higher in the tumor, but lower in the blood, in responders following virotherapy, suggesting that virus treatment may expand intratumoral T-cell clones that recognize tumor and/or viral antigens. Increased TCR diversity in the blood was suggestive of a systemic immune response. .
Conclusions: These clinical and translational findings support the further development of oncolytic virotherapy/ILP combinations to activate both systemic and local antitumor immunity, including in tumor types such as sarcoma, which are largely refractory to current treatment with immunotherapy VSports最新版本. .
Keywords: Abscopal; Oncolytic virus; Solid tumor; Surgery. V体育平台登录.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions VSports注册入口. Published by BMJ Group. .
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures
References
-
- Eggermont AM, Schraffordt Koops H, Klausner JM, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg. 1996;224:756–64. doi: 10.1097/00000658-199612000-00011. - V体育2025版 - DOI - PMC - PubMed
-
- Smith HG, Hayes AJ. The role of regional chemotherapy in the management of extremity soft tissue malignancies. Eur J Surg Oncol. 2016;42:7–17. doi: 10.1016/j.ejso.2015.08.165. - "V体育ios版" DOI - PubMed
-
- Deroose JP, Eggermont AMM, van Geel AN, et al. Long-term results of tumor necrosis factor alpha- and melphalan-based isolated limb perfusion in locally advanced extremity soft tissue sarcomas. J Clin Oncol. 2011;29:4036–44. doi: 10.1200/JCO.2011.35.6618. - V体育官网入口 - DOI - PubMed
"VSports注册入口" Publication types
- "VSports在线直播" Actions
MeSH terms
- Actions (V体育平台登录)
- Actions (VSports app下载)
- "V体育ios版" Actions
- VSports app下载 - Actions
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- Actions (VSports)
- Actions (V体育平台登录)
Substances
- Actions (V体育官网入口)
LinkOut - more resources
Full Text Sources
"V体育安卓版" Medical
