Exploring the dynamic relationship between antimicrobial resistance, virulence fitness, and host responses in Listeria monocytogenes infections
- PMID: 40987764
- PMCID: PMC12457630
- DOI: 10.1038/s41598-025-10093-z
Exploring the dynamic relationship between antimicrobial resistance, virulence fitness, and host responses in Listeria monocytogenes infections
Abstract
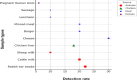
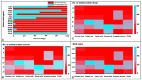
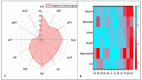
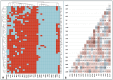
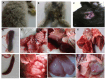
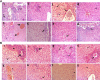
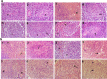
Listeriosis is a severe zoonotic disease caused by Listeria monocytogenes (L. monocytogenes), which can be acquired through animal source foods. This pathogen shows unique virulence fitness allowing it to penetrate and survive inside host cells causing extremely dangerous symptoms. Therefore, we aimed to correlate the clinical outcomes with the antimicrobial resistance and virulence profiles of L. monocytogenes. This is crucial for improving disease management, developing new therapeutic strategies, enhancing public health and food safety, and advancing scientific knowledge. Therefore, we assessed the antimicrobial resistance and virulence profiles of L. monocytogenes isolated from various sources, along with their potential to cause disease, using an in vivo rabbit model. Based on identification criteria, 47 L. monocytogenes isolates (15. 7%) were recovered with the highest detection rates among rabbits (22%). Unfortunately, all the investigated isolates showed multidrug resistance (MDR) as well as multi-virulent profiles with 45 highly heterogeneous clusters. Noteworthy, the degree of illnesses of the experimentally infected rabbits was dependent on the virulence profiles. Specific nervous manifestations and severe histopathological alterations were observed in experimental rabbits infected with highly virulent isolates confirming that the potential ability of this pathogen to produce a disease is not always decipherable from its virulence arrays. Finally, it was confirmed that managing infections caused by L. monocytogenes has become increasingly challenging due to its high antimicrobial resistance, strong virulence, and the severe pathological effects linked to its virulence factors. VSports手机版.
Keywords: L. monocytogenes; Pathogenicity; Rabbits; Resistance; Virulence; Zoonotic. V体育安卓版.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the ethical principles set forth by the Research Ethics Committee of Egypt’s Suez Canal University. The Ethics Review Committee (AERC-SCU) approved both the animal handling procedures and the collection of human samples under approval number SCU2023070. Consent for publication: were applicable and available up on demand. Competing interests: The authors declare no competing interests V体育ios版.
Figures

References
-
- Swaminathan, B. & Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect.9 (10), 1236–1243. 10.1016/j.micinf.2007.05.011 (2007). - PubMed
-
- Hoelzer, K., Pouillot, R. & Dennis, S. Animal models of listeriosis: a comparative review of the current state of the Art and lessons learned. Vet. Res.43 (1), 18. 10.1186/1297-9716-43-18 (2012). Published 2012 Mar 14. - PMC (VSports在线直播) - PubMed
-
- Morin, D. E. Brainstem and cranial nerve abnormalities: listeriosis, otitis media/interna, and pituitary abscess syndrome. Vet. Clin. North. Am. Food Anim. Pract.20 (2), 243–vi. 10.1016/j.cvfa.2004.02.007 (2004). - PubMed
-
- Van Metre, D. C., Barrington, G. M., Parish, S. M. & Tumas, D. B. Otitis media/interna and suppurative meningoencephalomyelitis associated with Listeria monocytogenes infection in a Llama. J. Am. Vet. Med. Assoc.199 (2), 236–240 (1991). - PubMed
MeSH terms
- "V体育2025版" Actions
- V体育官网 - Actions
- VSports注册入口 - Actions
- "V体育2025版" Actions
- VSports在线直播 - Actions
- "V体育ios版" Actions
- VSports最新版本 - Actions
- "V体育ios版" Actions
Substances
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"VSports最新版本" Medical

