doi: 10.3324/haematol.2024.287047.
Epub 2025 Apr 17.
Novel PROTAC to target FKBP12: the potential to enhance bone morphogenetic protein activity and apoptosis in multiple myeloma
Affiliations
- PMID: 40241574
- PMCID: PMC12485307 (V体育官网入口)
- DOI: 10.3324/haematol.2024.287047
Item in Clipboard
Novel PROTAC to target FKBP12: the potential to enhance bone morphogenetic protein activity and apoptosis in multiple myeloma (V体育ios版)
Haematologica.
.
No abstract available
Figures
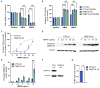
Depletion of FKBP12 enhanced bone morphogenetic protein-induced apoptosis in myeloma cells. (A) INA-6 CTR knockout (KO) and FKBP1A KO were made using clustered regularly interspaced short palindromic repeats (CRISPR) single guide (sg) RNA targeting FKBP1A (GGGCGCACCTTCCCCAAGCG) and an irrelevant, targeting control (CTR), sgRNA specific for intron 2 of UNG (CGACCCGCGAGATGATATCA, a kind gift from Per Arne Aas, NTNU, Trondheim, Norway). The sgRNA oligo pairs were ligated into lentiCRISPR v2 (a gift from Feng Zhang; Addgene plasmid #52961; RRID:Addgene_52961). The KO cells were treated with bone morphogenetic protein (BMP)4 (100 ng/mL) or BMP6 (7.5 ng/mL) and FK506 (100 nM) for 72 hours (hr) before cell viability was measured using a CellTiter-Glo luciferase assay. (B) Cells were treated for 48 hr with BMP4 (100 ng/mL) or BMP6 (7.5 ng/mL) and FK506 (100 nM), and caspase activity was measured using Caspase-Glo 3/7 Reagent (Promega). The result is shown as relative luciferase units. (A and B) Two-way ANOVA and Tukey multiple comparisons test were used to test significance. ***P<0.001, ****P<0.0001, ns: not significant. (C) INA-6 CTR KO and FKBP1A KO were treated for 72 hr with BMP6. The cells were stained with annexin V and propidium iodide (PI) using Apotest Annexin A5-FITC kit (VPS Diagnostics, Hoeven, the Netherlands). The average % of cells that were positive (pos) for annexin V or PI were plotted relative to medium control (0%) with error bars representing standard error of the mean (SEM). (D) The phospho-SMAD1/5 protein levels were measured in INA-6 CTR KO and INA-6 FKBP1A KO treated for 1 hr with increasing doses of BMP6. The antibodies used were phospho-SMAD/5 (Ser463/465) (RRID: AB_491015, #9516) and β-actin (RRID: AB_2223172, #4970) both from Cell Signaling Technology, BioNordika AS, Oslo, Norway. (E) Graph representing signal intensities shown in (D) relative to β-actin. Two-way ANOVA and Šídák’s multiple comparisons test were used to test significance. **P<0.01. (F) FKBP12 protein levels were measured in INA-6 CTR KO and INA-6 FKBP1A KO using antibodies targeting FKBP12 (RRID: AB_2102847, #SC-133067, Santa Cruz, TX, USA) and β-actin. (G) Graph representing signal intensities shown in (F) relative to β-actin. Significance was calculated using a paired two-sided t test. *P<0.05. All graphs show the mean and SEM of 3 independent experiments.

FKBP12-targeting PROteolysis targeting chimeras degraded FKBP12 and enhanced bone morphogenetic protein (BMP)-6-induced signaling and cell death. (A) Co-crystal structure (PDB-ID 8chl) of a prototypical [4.3.1]bicyclic sulfonamide ligand (green sticks) bound to FKBP12 (gray surface). The pyridine moiety, which is replaced by a triazole in the PROteolysis targeting chimera (PROTAC) 5a1, is circled in orange and the exit vector for the linker is indicated by the orange arrow. The structure is derived from Purder et al. (B) The human myeloma cell line INA-6 was treated with FKBP12 PROTAC (1 or 100 nM) for 18 hours (hr) before Western blotting with primary antibodies FKBP12 (RRID: AB_2102847, #SC-133067, Santa Cruz, TX, USA), FKBP4 (RRID:AB_2797737, #11826), FKBP5 (RRID:AB_2797846, #12210) (Cell Signaling Technology, BioNordika AS, Oslo, Norway), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (RRID: AB_2107448, #Ab8245, Abcam, Cambridge, UK). The image represents one of 3 independent experiments. Densitometric analysis based on all 3 replicates can be found in Online Supplementary Figure S2A-C. (C) INA-6 BRE-luc cells (50,000/well) were seeded in RPMI with 0.1% bovine serum albumin and interleukin-6 (1 ng/mL) and treated with increasing doses of PROTAC alone (blue lines/circles) or with bone morphogenetic protein (BMP)6 (7.5 ng/mL, green lines/circles) for 18 hr. FK506 (100 nM) was included as an internal control both alone and with BMP6 (green or blue triangle). The luciferase activity was measured using BriteLiteTM Plus Luciferase Detection Reagent (PerkinElmer Inc.) and plotted relative to BMP6 alone. Cell lines, INA-6 (D), IH-1 (E), Karpas-417 (F), and DOHH-2 (G) were treated with increasing doses of the FKBP12 targeting PROTAC 5a1, 6b4, and RC32, combined with BMP6 (7.5 ng/mL) for 72 hr before measuring cell viability with CellTiter-Glo (Promega). The graphs show the average and standard error of the mean (SEM) for 3 independent experiments.
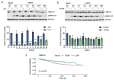
The effect of 5a1 on FKBP12 protein levels over time. INA-6 cells were treated for 18 hours (hr) with or without 5a1 (A) or FK506 (B). Some cells were subsequently kept as controls, and the remaining cells were washed with Hank’s balanced salt solution to remove the drugs. The cells were resuspended in experimental medium and incubated for the time-points indicated in the plot. Before harvesting, the cells were treated for 1 hr with bone morphogenetic protein (BMP)6 (7.5 ng/mL) to assess the effect of 5a1 and FK506 on FKBP12 protein levels and SMAD1/5 activity. Primary antibodies used were FKBP12 (RRID: AB_2102847, #SC-133067; Santa Cruz, CA, USA), phospho-SMAD1/5 (RRID: AB_491015, #9516; Cell Signaling Technology), and GAPDH (RRID: AB_2107448, #Ab8245, Abcam). One representative out of 3 independent experiments is shown. The graphs represent densitometric analysis of 3 independent experiments with the average and standard error of the mean (SEM). Two-way ANOVA and Šídák’s multiple comparisons test were used to test significance. *P<0.05, ***P<0.001, **** P<0.0001. (C) RNA-sequencing and clinical data of myeloma patients were obtained from the publicly available Multiple Myeloma Research Foundation (MMRF) CoMMpass database (https://research.themmrf.org). RNA-sequencing data of 762 patients were available. The MMRF-CoMMpass data was analyzed in R (2023.06.0+421) using TCGABiolinks and DESeq2. A Kaplan-Meyer plot was generated from the MMRF-CoMMpass dataset and shows overall survival (OS) related to FKBP1A expression (high>upper quarter percentile, low
References
-
- Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19(23):2783-2810. - PubMed
-
- Wang T, Li BY, Danielson PD, et al. The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell. 1996;86(3):435-444. - PubMed
-
- Holien T, Våtsveen TK, Hella H, et al. Bone morphogenetic proteins induce apoptosis in multiple myeloma cells by Smad-dependent repression of MYC. Leukemia. 2012;26(5):1073-1080. - PubMed (VSports注册入口)
LinkOut - more resources
Full Text Sources

