Inhibition of RIPK1 or RIPK3 kinase activity post ischemia-reperfusion reduces the development of chronic kidney injury
- PMID: 39705008
- PMCID: PMC12220529
- DOI: 10.1042/BCJ20240569
Inhibition of RIPK1 or RIPK3 kinase activity post ischemia-reperfusion reduces the development of chronic kidney injury
Abstract
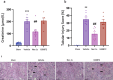
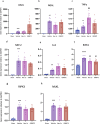
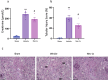
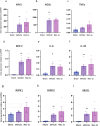
Ischemia-reperfusion injury (IRI) occurs when the blood supply to an organ is temporarily reduced and then restored. Kidney IRI is a form of acute kidney injury (AKI), which often progresses to kidney fibrosis VSports手机版. Necroptosis is a regulated necrosis pathway that has been implicated in kidney IRI. Necroptotic cell death involves the recruitment of the RIPK1 and RIPK3 kinases and the activation of the terminal effector, the mixed lineage kinase domain-like (MLKL) pseudokinase. Phosphorylated MLKL causes cell death by plasma membrane rupture, driving 'necroinflammation'. Owing to their apical role in the pathway, RIPK1 and RIPK3 have been implicated in the development of kidney fibrosis. Here, we used a mouse model of unilateral kidney IRI to assess whether the inhibition of RIPK1 or RIPK3 kinase activity reduces AKI and the progression to kidney fibrosis. Mice treated with the RIPK1 inhibitor Nec-1s, either before or after IR, showed reduced kidney injury at 24 hr compared with controls, whereas no protection was offered by the RIPK3 inhibitor GSK´872. In contrast, treatment with either inhibitor from days 3 to 9 post-IR reduced the degree of kidney fibrosis at day 28. These findings further support the role of necroptosis in IRI and provide important validation for the contribution of both RIPK1 and RIPK3 catalytic activities in the progression of kidney fibrosis. Targeting the necroptosis pathway could be a promising therapeutic strategy to mitigate kidney disease following IR. .
Keywords: acute kidney injury; ischemia-reperfusion; kidney fibrosis; kinase inhibitor injury; programmed necrosis. V体育安卓版.
© 2025 The Author(s).
Conflict of interest statement
All authors contribute, or have contributed, to a project developing necroptosis pathway inhibitors in collaboration with Anaxis Pharma Pty Ltd. JMM and PJC have received research funding from Anaxis Pharma Pty Ltd V体育ios版. FI is Chair of the SVHM Animal Ethics Committee, declared a conflict of interest and removed himself from any decision processes for the protocols 026/17 and 022/20 used in the present study.
Figures


"V体育ios版" References
-
- Kako K., Kato M., Matsuoka T., Mustapha A. Depression of membrane-bound Na+-K+-ATPase activity induced by free radicals and by ischemia of kidney. Am. J. Physiol. Cell Physiol. 1988;254:C330–C337. doi: 10.1152/ajpcell.1988.254.2.C330. - DOI (V体育平台登录) - PubMed
Publication types (V体育官网)
- V体育安卓版 - Actions
MeSH terms
- V体育安卓版 - Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- "VSports app下载" Actions
- Actions (V体育ios版)
- V体育官网入口 - Actions
- Actions (V体育ios版)
- "V体育安卓版" Actions
- "VSports注册入口" Actions
- "VSports" Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- VSports - Actions
Substances
- V体育官网入口 - Actions
- VSports注册入口 - Actions
- Actions (V体育官网入口)
- Actions (VSports注册入口)
LinkOut - more resources
Full Text Sources
"VSports在线直播" Medical
Miscellaneous

