Efficacy, safety, and survival of neoadjuvant immunochemotherapy in operable non-small cell lung cancer: a systematic review and meta-analysis
- PMID: 38106421
- PMCID: PMC10722296
- DOI: V体育安卓版 - 10.3389/fimmu.2023.1273220
Efficacy, safety, and survival of neoadjuvant immunochemotherapy in operable non-small cell lung cancer: a systematic review and meta-analysis
Abstract
Background: Neoadjuvant immunochemotherapy may benefit patients with non-small cell lung cancer (NSCLC), but its impact requires further investigation VSports手机版. .
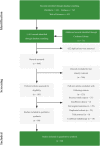
Methods: A meta-analysis was conducted. PubMed, Embase, Web of Science, and the Cochrane Library were searched. The study was registered in PROSPERO (registration no. CRD42022360893) V体育安卓版. .
Results: 60 studies of 3,632 patients were included. Comparing with neoadjuvant chemotherapy, neoadjuvant immunochemotherapy showed higher pCR (RR: 4. 71, 95% CI: 3. 69, 6. 02), MPR (RR, 3. 20, 95% CI: 2. 75, 3. 74), and ORR (RR, 1. 46, 95% CI: 1. 21, 1. 77), fewer surgical complications (RR: 0. 67, 95%CI: 0. 48, 0. 94), higher R0 resection rate (RR: 1. 06, 95%CI: 1. 03, 1. 10, I2 = 52%), and longer 1-year and 2-year OS, without affecting TRAEs. For neoadjuvant immunochemotherapy in NSCLC, the pooled pCR rate was 0. 35 (95% CI: 0. 31, 0. 39), MPR was 0. 59 (95% CI: 0. 54, 0. 63), and ORR was 0. 71 (95% CI: 0. 66, 0. 76). The pooled incidence of all grade TRAEs was 0. 70 (95% CI: 0. 60, 0. 81), and that of >= grade 3 TRAEs was 0. 24 (95% CI: 0. 16, 0. 32). The surgical complications rate was 0. 13 (95% CI: 0. 07, 0. 18) and R0 resection rate was 0. 98 (95% CI: 0. 96, 0. 99). The pooled 1-year OS was 0. 97 (95%CI: 0. 96, 0. 99), and 2-year OS was 0. 89 (95%CI: 0. 83, 0. 94). Patients with squamous cell carcinoma, stage III or higher PD-L1 performed better. Notably, no significant differences were observed in pCR, MPR, and ORR between 2 or more treatment cycles. Pembrolizumab-, or toripalimab-based neoadjuvant immunochemotherapy demonstrated superior efficacy and tolerable toxicity. V体育ios版.
Conclusion: According to our analysis, reliable efficacy, safety, and survival of neoadjuvant immunochemotherapy for operable NSCLC were demonstrated VSports最新版本. .
Systematic review registration: https://www. crd. york. ac. uk/prospero/display_record. php. ID=CRD42022360893, identifier CRD42022360893. V体育平台登录.
Keywords: efficacy; neoadjuvant immunochemotherapy; non-small cell lung cancer; safety; survival VSports注册入口. .
Copyright © 2023 Zheng, Feng, Chen and You. V体育官网入口.
Conflict of interest statement (VSports手机版)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
"VSports app下载" Figures




References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708 - VSports手机版 - DOI - PubMed
"V体育平台登录" Publication types
- Actions (VSports手机版)
- "V体育ios版" Actions
MeSH terms
- Actions (VSports注册入口)
- V体育官网 - Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials

