Angiotensin II receptor inhibition ameliorates liver fibrosis and enhances hepatocellular carcinoma infiltration by effector T cells
- PMID: 37126700
- PMCID: PMC10175751
- DOI: 10.1073/pnas.2300706120
Angiotensin II receptor inhibition ameliorates liver fibrosis and enhances hepatocellular carcinoma infiltration by effector T cells
V体育2025版 - Abstract
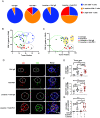
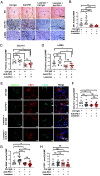
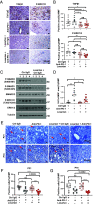
Although viral hepatocellular carcinoma (HCC) is declining, nonviral HCC, which often is the end stage of nonalcoholic or alcoholic steatohepatitis (NASH, ASH), is on an upward trajectory. Immune checkpoint inhibitors (ICIs) that block the T cell inhibitory receptor PD-1 were approved for treatment of all HCC types. However, only a minority of HCC patients show a robust and sustained response to PD-1 blockade, calling for improved understanding of factors that negatively impact response rate and duration and the discovery of new adjuvant treatments that enhance ICI responsiveness. Using a mouse model of NASH-driven HCC, we identified peritumoral fibrosis as a potential obstacle to T cell-mediated tumor regression and postulated that antifibrotic medications may increase ICI responsiveness. We now show that the angiotensin II receptor inhibitor losartan, a commonly prescribed and safe antihypertensive drug, reduced liver and peritumoral fibrosis and substantially enhanced anti-PD-1-induced tumor regression VSports手机版. Although losartan did not potentiate T cell reinvigoration, it substantially enhanced HCC infiltration by effector CD8+ T cells compared to PD-1 blockade alone. The beneficial effects of losartan correlated with blunted TGF-β receptor signaling, reduced collagen deposition, and depletion of immunosuppressive fibroblasts. .
Keywords: NASH-driven HCC; anti-PD-1; liver fibrosis; losartan V体育安卓版. .
Conflict of interest statement
M. K. is the founder and stockholder in Elgia Pharmaceuticals V体育ios版. All other authors declare no competing interest.
VSports在线直播 - Figures

Update of
-
"VSports手机版" Angiotensin II receptor inhibition ameliorates liver fibrosis and enhances hepatocellular carcinoma infiltration by effector T cells.bioRxiv [Preprint]. 2023 Mar 6:2023.03.05.531188. doi: 10.1101/2023.03.05.531188. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 May 9;120(19):e2300706120. doi: 10.1073/pnas.2300706120. PMID: 36945365 Free PMC article. Updated. Preprint.
References
-
- Llovet J. M., et al. , Hepatocellular carcinoma. Nat. Rev. Dis. Primers 7, 6 (2021). - PubMed
Publication types
- Actions (VSports在线直播)
"V体育2025版" MeSH terms
- V体育官网 - Actions
- "V体育官网" Actions
- V体育安卓版 - Actions
- "VSports注册入口" Actions
- Actions (VSports手机版)
- Actions (V体育2025版)
Substances (V体育2025版)
- Actions (VSports手机版)
Grants and funding
"V体育官网入口" LinkOut - more resources
Full Text Sources (V体育安卓版)
Medical
Research Materials
Miscellaneous

