Enterocyte-specific deletion of metal transporter Zip14 (Slc39a14) alters intestinal homeostasis through epigenetic mechanisms
- PMID: 36537699
- PMCID: PMC9925170
- DOI: V体育平台登录 - 10.1152/ajpgi.00244.2022
"V体育安卓版" Enterocyte-specific deletion of metal transporter Zip14 (Slc39a14) alters intestinal homeostasis through epigenetic mechanisms
Abstract
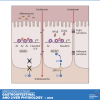
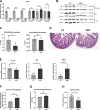
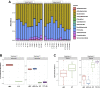
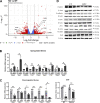
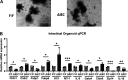
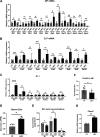
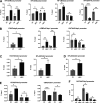
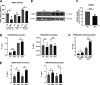
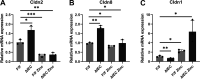
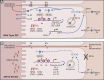
Zinc has anti-inflammatory properties using mechanisms that are unclear. Zip14 (Slc39a14) is a zinc transporter induced by proinflammatory stimuli and is highly expressed at the basolateral membrane of intestinal epithelial cells (IECs). Enterocyte-specific Zip14 ablation (Zip14ΔIEC) in mice was developed to study the functions of this transporter in enterocytes. This gene deletion led to increased intestinal permeability, increased IL-6 and IFNγ expression, mild endotoxemia, and intestinal dysbiosis. RNA sequencing was used for transcriptome profiling. These analyses revealed differential expression of specific intestinal proinflammatory and tight junction (TJ) genes. Binding of transcription factors, including NF-κβ, STAT3, and CDX2, to appropriate promoter sites of these genes supports the differential expression shown with chromatin immunoprecipitation assays. Total histone deacetylase (HDAC), and specifically HDAC3, activities were markedly reduced with Zip14 ablation. Intestinal organoids derived from ΔIEC mice display TJ and cytokine gene dysregulation compared with control mice VSports手机版. Differential expression of specific genes was reversed with zinc supplementation of the organoids. We conclude that zinc-dependent HDAC enzymes acquire zinc ions via Zip14-mediated transport and that intestinal integrity is controlled in part through epigenetic modifications. NEW & NOTEWORTHY We show that enterocyte-specific ablation of zinc transporter Zip14 (Slc39a14) results in selective dysbiosis and differential expression of tight junction proteins, claudin 1 and 2, and specific cytokines associated with intestinal inflammation. HDAC activity and zinc uptake are reduced with Zip14 ablation. Using intestinal organoids, the expression defects of claudin 1 and 2 are resolved through zinc supplementation. These novel results suggest that zinc, an essential micronutrient, influences gene expression through epigenetic mechanisms. .
Keywords: Zip14; cytokines; inflammation; intestinal; zinc. V体育安卓版.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
"VSports最新版本" Figures
VSports app下载 - References
-
- Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 103: 13612–13617, 2006. doi:10.1073/pnas.0606424103. - "V体育官网" DOI - PMC - PubMed
Publication types
- "VSports最新版本" Actions
"V体育平台登录" MeSH terms
- Actions (V体育安卓版)
- Actions (VSports app下载)
- Actions (VSports手机版)
- Actions (VSports注册入口)
- V体育2025版 - Actions
- Actions (VSports在线直播)
- "VSports" Actions
Substances
- "V体育平台登录" Actions
- Actions (VSports在线直播)
Grants and funding
"VSports在线直播" LinkOut - more resources
Full Text Sources
Molecular Biology Databases (V体育ios版)
Miscellaneous

