VSports最新版本 - Improved Bioavailability of Ebastine through Development of Transfersomal Oral Films
- PMID: 34452276
- PMCID: PMC8401636
- DOI: VSports app下载 - 10.3390/pharmaceutics13081315
"VSports" Improved Bioavailability of Ebastine through Development of Transfersomal Oral Films
Abstract
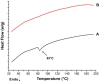
The main objective of this research work was the development and evaluation of transfersomes integrated oral films for the bioavailability enhancement of Ebastine (EBT) to treat allergic rhinitis. The flexible transfersomes, consisting of drug (EBT), lipid (Phosphatidylcholine) and edge activator (EA) Polyoxyethylene sorbitan monooleate or Sorbitan monolaurate, were prepared with the conventional thin film hydration method. The developed transfersomes were further integrated into oral films using the solvent casting method. Transfersomes were evaluated for their size distribution, surface charge, entrapment efficiency (EE%) and relative deformability, whereas the formulated oral films were characterized for weight, thickness, pH, folding endurance, tensile strength, % of elongation, degree of crystallinity, water content, content uniformity, in vitro drug release and ex vivo permeation, as well as in vivo pharmacokinetic and pharmacodynamics profile. The mean hydrodynamic diameter of transfersomes was detected to be 75. 87 ± 0. 55 nm with an average PDI and zeta potential of 0. 089 ± 0. 01 and 33. 5 ± 0. 39 mV, respectively. The highest deformability of transfersomes of 18. 52 mg/s was observed in the VS-3 formulation. The average entrapment efficiency of the transfersomes was about 95. 15 ± 1. 4%. Transfersomal oral films were found smooth with an average weight, thickness and tensile strength of 174. 72 ± 2. 3 mg, 0. 313 ± 0. 03 mm and 36. 4 ± 1. 1 MPa, respectively. The folding endurance, pH and elongation were found 132 ± 1, 6. 8 ± 0. 2 and 10 VSports手机版. 03 ± 0. 4%, respectively. The ex vivo permeability of EBT from formulation ETF-5 was found to be approximately 2. 86 folds higher than the pure drug and 1. 81 folds higher than plain film (i. e. , without loaded transfersomes). The relative oral bioavailability of ETF-5 was 2. 95- and 1. 7-fold higher than that of EBT-suspension and plain film, respectively. In addition, ETF-5 suppressed the wheal and flare completely within 24 h. Based on the physicochemical considerations, as well as in vitro and in vivo characterizations, it is concluded that the highly flexible transfersomal oral films (TOFs) effectively improved the bioavailability and antihistamine activity of EBT. .
Keywords: bioavailability; ebastine; edge activator; in vivo; phospholipids; transfersomes. V体育安卓版.
Conflict of interest statement (VSports最新版本)
The authors declare no conflict of interest.
Figures









References (VSports在线直播)
-
- Antonijoan R., García-Gea C., Puntes M., Pérez J., Esbrí R., Serra C., Fortea J., Barbanoj M.J. Comparison of inhibition of cutaneous histamine reaction of ebastine fast-dissolving tablet (20 mg) versus desloratadine capsule (5 mg): A randomized, double-blind, double-dummy, placebo-controlled, three-period crossover study in healthy, nonatopic adults. Clin. Ther. 2007;29:814–822. doi: 10.1016/j.clinthera.2007.05.001. - DOI - PubMed
-
- Dierick B.J., van der Molen T., Flokstra-de Blok B.M., Muraro A., Postma M.J., Kocks J.W., van Boven J.F. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharm. Outcomes Res. 2020;20:437–453. doi: 10.1080/14737167.2020.1819793. - V体育官网入口 - DOI - PubMed
-
- Sastre J. Ebastine in the treatment of allergic rhinitis and urticaria: 30 years of clinical studies and real-world experience. J. Investig. Allergol. Clin. Immunol. 2020;30:156–168. doi: 10.18176/jiaci.0401. - "V体育安卓版" DOI - PubMed
-
- Islam N., Zahoor A.F., Syed H.K., Iqbal M.S., Khan I.U., Abbas G., Mushtaq M., Rehman M.U., Rasul A., Ikram M. Improvement of solubility and dissolution of ebastine by fabricating phosphatidylcholine/bile salt bilosomes. Pak. J. Pharm. Sci. 2020;33:2301–2306. - PubMed
LinkOut - more resources
Full Text Sources

