"VSports手机版" The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study
- PMID: 34258590
- PMCID: PMC8266273
- DOI: 10.1016/S2665-9913(21)00212-5
The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study (VSports手机版)
Abstract
Background: Patients on therapeutic immunosuppressants for immune-mediated inflammatory diseases were excluded from COVID-19 vaccine trials. We therefore aimed to evaluate humoral and cellular immune responses to COVID-19 vaccine BNT162b2 (Pfizer-BioNTech) in patients taking methotrexate and commonly used targeted biological therapies, compared with healthy controls. Given the roll-out of extended interval vaccination programmes to maximise population coverage, we present findings after the first dose VSports手机版. .
Methods: In this cohort study, we recruited consecutive patients with a dermatologist-confirmed diagnosis of psoriasis who were receiving methotrexate or targeted biological monotherapy (tumour necrosis factor [TNF] inhibitors, interleukin [IL]-17 inhibitors, or IL-23 inhibitors) from a specialist psoriasis centre serving London and South East England. Consecutive volunteers without psoriasis and not receiving systemic immunosuppression who presented for vaccination at Guy's and St Thomas' NHS Foundation Trust (London, UK) were included as the healthy control cohort. All participants had to be eligible to receive the BNT162b2 vaccine. Immunogenicity was evaluated immediately before and on day 28 (±2 days) after vaccination. The primary outcomes were humoral immunity to the SARS-CoV-2 spike glycoprotein, defined as neutralising antibody responses to wild-type SARS-CoV-2, and spike-specific T-cell responses (including interferon-γ, IL-2, and IL-21) 28 days after vaccination. V体育安卓版.
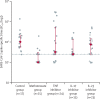
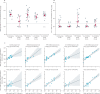
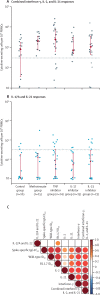
Findings: Between Jan 14 and April 4, 2021, 84 patients with psoriasis (17 on methotrexate, 27 on TNF inhibitors, 15 on IL-17 inhibitors, and 25 on IL-23 inhibitors) and 17 healthy controls were included. The study population had a median age of 43 years (IQR 31-52), with 56 (55%) males, 45 (45%) females, and 85 (84%) participants of White ethnicity. Seroconversion rates were lower in patients receiving immunosuppressants (60 [78%; 95% CI 67-87] of 77) than in controls (17 [100%; 80-100] of 17), with the lowest rate in those receiving methotrexate (seven [47%; 21-73] of 15). Neutralising activity against wild-type SARS-CoV-2 was significantly lower in patients receiving methotrexate (median 50% inhibitory dilution 129 [IQR 40-236]) than in controls (317 [213-487], p=0·0032), but was preserved in those receiving targeted biologics (269 [141-418]). Neutralising titres against the B V体育ios版. 1. 1. 7 variant were similarly low in all participants. Cellular immune responses were induced in all groups, and were not attenuated in patients receiving methotrexate or targeted biologics compared with controls. .
Interpretation: Functional humoral immunity to a single dose of BNT162b2 is impaired by methotrexate but not by targeted biologics, whereas cellular responses are preserved. Seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression. Real-world pharmacovigilance studies will determine how these findings reflect clinical effectiveness. VSports最新版本.
Funding: UK National Institute for Health Research. V体育平台登录.
© 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4. 0 license. VSports注册入口.
Conflict of interest statement
CHS reports grants from AbbVie, Sanofi, Novartis, and Pfizer, outside the submitted work. JBG reports personal fees from AbbVie, Sanofi, Novartis, Pfizer, Janssen, and UCB, and grants from Eli Lilly, outside the submitted work. SKM reports departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sanofi, and UCB, outside the submitted work. MAB reports departmental income from Clementia, Eli Lilly, Ipsen, Novartis, Pathios Therapeutics, Regeneron, and UCB, outside the submitted work V体育官网入口. JNB reports grants and personal fees from AbbVie, Novartis, Lilly, and J&J, outside the submitted work. SN reports personal fees from Pfizer and Janssen, outside of the submitted work. APC reports grants from Bristol Myers Squibb and Janssen, and speaker bureau and consultancy fees from AbbVie, Bristol Myers Squibb, and UCB. TIMT reports grants from GlaxoSmithKline, Sanofi, and Imcyse, and consultancy fees from GlaxoSmithKline, Novartis, UCB, and Quell Therapeutics, outside the submitted work. All other authors declare no competing interests.
"VSports手机版" Figures
References
-
- Safiri S, Kolahi AA, Hoy D. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78:1463–1471. - PubMed
-
- Mehrmal S, Uppal P, Nedley N, Giesey RL, Delost GR. The global, regional, and national burden of psoriasis in 195 countries and territories, 1990 to 2017: a systematic analysis from the Global Burden of Disease Study 2017. J Am Acad Dermatol. 2021;84:46–52. - PubMed
-
- GBD 2017 Inflammatory Bowel Disease Collaborators The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. - VSports - PMC - PubMed
-
- Kalb RE, Fiorentino DF, Lebwohl MG. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) JAMA Dermatol. 2015;151:961–969. - PubMed (VSports)
-
- Yiu ZZN, Ashcroft DM, Evans I. Infliximab is associated with an increased risk of serious infection in patients with psoriasis in the U.K. and Republic of Ireland: results from the British Association of Dermatologists Biologic Interventions Register (BADBIR) Br J Dermatol. 2019;180:329–337. - PMC - PubMed
Grants and funding
V体育ios版 - LinkOut - more resources
Full Text Sources
Miscellaneous

