"V体育平台登录" Propelling Healthcare with Advanced Therapy Medicinal Products: A Policy Discussion
- PMID: 33987187
- PMCID: "VSports app下载" PMC8101061
- DOI: 10.1159/000511678
Propelling Healthcare with Advanced Therapy Medicinal Products: A Policy Discussion
Abstract
Recent advances in biomedicine are opening the door to new approaches, and treatment and prevention are being transformed by novel medicines based on genetic engineering, innovative cell-based therapies and tissue-engineered products, and combinations of a medical device with embedded cell or tissue components. These advanced therapy medicinal products (ATMPs) hold one of the keys to making a reality of genuinely personalised medicine. There are an estimated 450 companies across the globe working on the development of gene therapies and more than 1,000 clinical trials underway worldwide, and some 20-30 new ATMPs filings are expected in Europe annually over the next 5 years. But challenges confront the sector, complicating the translation from research into patient access. Scientific, clinical development and regulatory issues are compounded by limited experience with clinical and commercial use, limited manufacturing know-how, high costs, and difficulties in accessing development funding and investment VSports手机版. Pricing and reimbursement and market access issues are an additional challenge, particularly in Europe, where unfamiliarity with the technology and uncertainty over the use of real-world evidence induce caution among clinicians, health technology assessment bodies and payers. There is a need for a review of the suitability of the regulatory and market access framework for these products, focused development of data, public/private partnerships, and fuller collaboration governments, doctors, insurers, patients, and pharmaceutical companies. This paper makes specific recommendations for all stakeholders, ranging from early dialogue on potential products, linking of clinical data and patient registries or standardisation of control frameworks, to a comprehensive approach to evidence generation, assessment, pricing, and payment for ATMPs. .
Keywords: European Union; Gene therapy; Gene transfer; Personalised healthcare; Personalised medicine; Policy; Quality of life; Tissue engineering; Vector design; Virus V体育安卓版. .
Copyright © 2020 by S. Karger AG, Basel.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




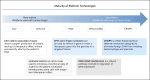
References
-
- Yu TT, Gupta P, Ronfard V, Vertès AA, Bayon Y. Recent Progress in European Advanced Therapy Medicinal Products and Beyond. Front Bioeng Biotechnol. 2018 Sep;6:130. - "VSports" PMC - PubMed
-
- EMA Regulatory Science Strategy to 2025. Cited June 23, 2020. Available from: http://www.ema.europa.eu/en/annual-report-2019/regulatory-science-strate....
-
- Gancberg D. Twenty Years of European Union Support to Gene Therapy and Gene Transfer. Hum Gene Ther. 2017 Nov;28((11)):951–3. - PubMed
-
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000 Apr;288((5466)):669–72. - "VSports在线直播" PubMed
-
- Mullard A. EMA greenlights second gene therapy. Nat Rev Drug Discov. 2016 May;15((5)):299. - PubMed

