"V体育官网入口" Update on New Aspects of the Renin-Angiotensin System in Hepatic Fibrosis and Portal Hypertension: Implications for Novel Therapeutic Options
- PMID: 33670126
- PMCID: PMC7916881
- DOI: 10.3390/jcm10040702
Update on New Aspects of the Renin-Angiotensin System in Hepatic Fibrosis and Portal Hypertension: Implications for Novel Therapeutic Options
Abstract (VSports手机版)
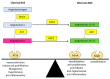
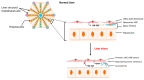
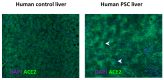
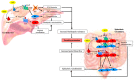
There is considerable experimental evidence that the renin angiotensin system (RAS) plays a central role in both hepatic fibrogenesis and portal hypertension VSports手机版. Angiotensin converting enzyme (ACE), a key enzyme of the classical RAS, converts angiotensin I (Ang I) to angiotensin II (Ang II), which acts via the Ang II type 1 receptor (AT1R) to stimulate hepatic fibrosis and increase intrahepatic vascular tone and portal pressure. Inhibitors of the classical RAS, drugs which are widely used in clinical practice in patients with hypertension, have been shown to inhibit liver fibrosis in animal models but their efficacy in human liver disease is yet to be tested in adequately powered clinical trials. Small trials in cirrhotic patients have demonstrated that these drugs may lower portal pressure but produce off-target complications such as systemic hypotension and renal failure. More recently, the alternate RAS, comprising its key enzyme, ACE2, the effector peptide angiotensin-(1-7) (Ang-(1-7)) which mediates its effects via the putative receptor Mas (MasR), has also been implicated in the pathogenesis of liver fibrosis and portal hypertension. This system is activated in both preclinical animal models and human chronic liver disease and it is now well established that the alternate RAS counter-regulates many of the deleterious effects of the ACE-dependent classical RAS. Work from our laboratory has demonstrated that liver-specific ACE2 overexpression reduces hepatic fibrosis and liver perfusion pressure without producing off-target effects. In addition, recent studies suggest that the blockers of the receptors of alternate RAS, such as the MasR and Mas related G protein-coupled receptor type-D (MrgD), increase splanchnic vascular resistance in cirrhotic animals, and thus drugs targeting the alternate RAS may be useful in the treatment of portal hypertension. This review outlines the role of the RAS in liver fibrosis and portal hypertension with a special emphasis on the possible new therapeutic approaches targeting the ACE2-driven alternate RAS. .
Keywords: Mas related G protein-coupled receptor type-D; angiotensin converting enzyme 2; angiotensin-(1–7); liver fibrosis and cirrhosis; portal hypertension; renin angiotensin system. V体育安卓版.
"V体育平台登录" Conflict of interest statement
The authors declare no conflict of interest.
Figures (VSports在线直播)
References
-
- Schuppan D., Afdhal N.H. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. - V体育平台登录 - DOI - PMC - PubMed
-
- WHO . Summary Tables of Mortality Estimates by Cause, Age and Sex, Globally and by Region, 2000–2016. WHO; Geneva, Switzerland: 2019.
Publication types
LinkOut - more resources
V体育官网入口 - Full Text Sources
Other Literature Sources (V体育ios版)
"VSports app下载" Miscellaneous

