Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies
- PMID: 33548172
- PMCID: PMC7936855
- DOI: 10.1016/j.cell.2021.01.016
"VSports最新版本" Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies
Abstract
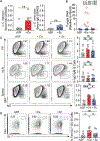
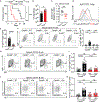
Antibodies mediate natural and vaccine-induced immunity against viral and bacterial pathogens, whereas fungi represent a widespread kingdom of pathogenic species for which neither vaccine nor neutralizing antibody therapies are clinically available VSports手机版. Here, using a multi-kingdom antibody profiling (multiKAP) approach, we explore the human antibody repertoires against gut commensal fungi (mycobiota). We identify species preferentially targeted by systemic antibodies in humans, with Candida albicans being the major inducer of antifungal immunoglobulin G (IgG). Fungal colonization of the gut induces germinal center (GC)-dependent B cell expansion in extraintestinal lymphoid tissues and generates systemic antibodies that confer protection against disseminated C. albicans or C. auris infection. Antifungal IgG production depends on the innate immunity regulator CARD9 and CARD9+CX3CR1+ macrophages. In individuals with invasive candidiasis, loss-of-function mutations in CARD9 are associated with impaired antifungal IgG responses. These results reveal an important role of gut commensal fungi in shaping the human antibody repertoire through CARD9-dependent induction of host-protective antifungal IgG. .
Keywords: B cells; CARD9; CX3CR1 macrophages; Candida albicans; Candida auris; antifungal IgG antibodies; gut fungi; gut-disseminated fungal infections; invasive candidiasis; mycobiome V体育安卓版. .
Copyright © 2021 Elsevier Inc V体育ios版. All rights reserved. .
"VSports最新版本" Conflict of interest statement
Declaration of interests Cornell University has filed a provisional patent application covering inventions described in this manuscript.
Figures




Comment in
-
Local farming of gut fungi protects against dangerous imports.Cell Host Microbe. 2021 Mar 10;29(3):311-312. doi: 10.1016/j.chom.2021.02.014. Cell Host Microbe. 2021. PMID: 33705697 Free PMC article.
-
"V体育ios版" Gut mycobiota modulates antifungal antibody-mediated immunity.Nat Rev Gastroenterol Hepatol. 2021 Apr;18(4):215. doi: 10.1038/s41575-021-00439-z. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33707767 No abstract available.
References (V体育官网)
-
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M, et al.; MetaHIT Consortium (2011). Enterotypes of the human gut microbiome. Nature 473, 174–180. - "VSports" PMC - PubMed
-
- Bacher P, Hohnstein T, Beerbaum E, Röcker M, Blango MG, Kaufmann S, Röhmel J, Eschenhagen P, Grehn C, Seidel K, et al. (2019). Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 176, 1340–1355.e15. - PubMed
-
- Barbet G, Sander LE, Geswell M, Leonardi I, Cerutti A, Iliev I, and Blander JM (2018). Sensing Microbial Viability through Bacterial RNA Augments T Follicular Helper Cell and Antibody Responses. Immunity 48, 584–598.e5. - VSports - PMC - PubMed
-
- Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, and White TC (2012). Hidden killers: human fungal infections. Sci. Transl. Med 4, 165rv13. - PubMed
"V体育平台登录" Publication types
- "VSports最新版本" Actions
MeSH terms
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- "V体育官网入口" Actions
- V体育安卓版 - Actions
- VSports - Actions
- "VSports注册入口" Actions
- Actions (VSports在线直播)
Substances
- "VSports app下载" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

