Salmonella versus the Microbiome (V体育官网)
- PMID: 33361269
- PMCID: PMC8549850
- DOI: 10.1128/MMBR.00027-19
Salmonella versus the Microbiome
Abstract
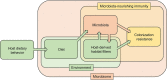
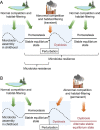
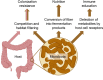
A balanced gut microbiota contributes to health, but the mechanisms maintaining homeostasis remain elusive. Microbiota assembly during infancy is governed by competition between species and by environmental factors, termed habitat filters, that determine the range of successful traits within the microbial community. These habitat filters include the diet, host-derived resources, and microbiota-derived metabolites, such as short-chain fatty acids. Once the microbiota has matured, competition and habitat filtering prevent engraftment of new microbes, thereby providing protection against opportunistic infections. Competition with endogenous Enterobacterales, habitat filtering by short-chain fatty acids, and a host-derived habitat filter, epithelial hypoxia, also contribute to colonization resistance against Salmonella serovars. However, at a high challenge dose, these frank pathogens can overcome colonization resistance by using their virulence factors to trigger intestinal inflammation. In turn, inflammation increases the luminal availability of host-derived resources, such as oxygen, nitrate, tetrathionate, and lactate, thereby creating a state of abnormal habitat filtering that enables the pathogen to overcome growth inhibition by short-chain fatty acids. Thus, studying the process of ecosystem invasion by Salmonella serovars clarifies that colonization resistance can become weakened by disrupting host-mediated habitat filtering. This insight is relevant for understanding how inflammation triggers dysbiosis linked to noncommunicable diseases, conditions in which endogenous Enterobacterales expand in the fecal microbiota using some of the same growth-limiting resources required by Salmonella serovars for ecosystem invasion VSports手机版. In essence, ecosystem invasion by Salmonella serovars suggests that homeostasis and dysbiosis simply represent states where competition and habitat filtering are normal or abnormal, respectively. .
Keywords: Salmonella; colonization resistance; microbiome; microbiota V体育安卓版. .
Copyright © 2020 American Society for Microbiology V体育ios版. .
Figures (V体育安卓版)




References
-
- Koch R. 1882. Die Aetiologie der Tuberkulose. Berliner Klin Wochenschr 15:221–230.
-
- Roux EYA. 1888. Contribution à l’étude de la diphtérie. Ann Inst Pasteur 2:421–499.
-
- Neisser M, Shiga K. 1903. Ueber freie Receptoren von Typhus- und Dysenteriebazillen und über das Dysenterietoxin. Dtsch Med Wochenschr 29:61–62. doi:10.1055/s-0028-1138255. - "V体育2025版" DOI
-
- Conradi H. 1903. Über lösliche, durch aseptische Autolyse erhaltene Giftstoffe von Ruhr- und Typhus-Bazillen. Dtsch Med Wochenschr 29:26–28. doi:10.1055/s-0028-1138228. - DOI
Publication types
- "V体育安卓版" Actions
- Actions (V体育官网)
- Actions (VSports注册入口)
MeSH terms
- V体育官网 - Actions
- "VSports在线直播" Actions
- Actions (VSports)
- "V体育2025版" Actions
- "VSports" Actions
Substances
- "V体育ios版" Actions
Grants and funding (VSports最新版本)
"V体育ios版" LinkOut - more resources
Full Text Sources
Other Literature Sources

