The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis
- PMID: 32562381
- PMCID: "V体育官网入口" PMC7305237
- DOI: 10.14814/phy2.14456
"V体育官网入口" The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis
Abstract
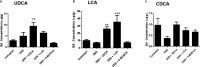
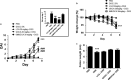
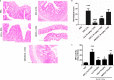
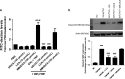
Increased epithelial permeability is a key feature of IBD pathogenesis and it has been proposed that agents which promote barrier function may be of therapeutic benefit. We have previously reported the secondary bile acid, ursodeoxycholic acid (UDCA), to be protective in a mouse model of colonic inflammation and that its bacterial metabolism is required for its beneficial effects. The current study aimed to compare the effects of UDCA, LCA, and a non-metabolizable analog of UDCA, 6-methyl-UDCA (6-MUDCA), on colonic barrier function and mucosal inflammation in a mouse model of colonic inflammation. Bile acids were administered daily to C57Bl6 mice by intraperitoneal injection. Colonic inflammation, induced by addition of DSS (2. 5%) to the drinking water, was measured as disease activity index (DAI) and histological score. Epithelial permeability and apoptosis were assessed by measuring FITC-dextran uptake and caspase-3 cleavage, respectively. Cecal bile acids were measured by HPLC-MS/MS. UDCA and LCA, but not 6-MUDCA, were protective against DSS-induced increases in epithelial permeability and colonic inflammation. Furthermore, UDCA and LCA inhibited colonic epithelial caspase-3 cleavage both in DSS-treated mice and in an in vitro model of cytokine-induced epithelial injury. HPLC-MS/MS analysis revealed UDCA administration to increase colonic LCA levels, whereas LCA administration did not alter UDCA levels. UDCA, and its primary metabolite, LCA, protect against intestinal inflammation in vivo, at least in part, by inhibition of epithelial apoptosis and promotion of barrier function. These data suggest that clinical trials of UDCA in IBD patients are warranted VSports手机版. .
Keywords: apoptosis; bile acid; colitis; epithelial barrier function. V体育安卓版.
© 2020 The Authors V体育ios版. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society. .
Conflict of interest statement
None declared.
Figures

References (VSports在线直播)
-
- Alemi, F. , Poole, D. P. , Chiu, J. , Schoonjans, K. , Cattaruzza, F. , Grider, J. R. , … Corvera, C. U. (2013). The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology, 144, 145–154. 10.1053/j.gastro.2012.09.055 - V体育ios版 - DOI - PMC - PubMed
-
- Araki, Y. , Andoh, A. , Tsujikawa, T. , Fujiyama, Y. , & Bamba, T. (2001). Alterations in intestinal microflora, faecal bile acids and short chain fatty acids in dextran sulphate sodium‐induced experimental acute colitis in rats. European Journal of Gastroenterology & Hepatology, 13(2), 107–112. 10.1097/00042737-200102000-00004 - DOI - PubMed
-
- Beuers, U. (2006). Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nature Clinical Practice Gastroenterology & Hepatology, 3, 318–328. - "VSports最新版本" PubMed
-
- Cabrera, D. , Arab, J. P. , & Arrese, M. (2019). UDCA, NorUDCA, and TUDCA in liver diseases: A review of their mechanisms of action and clinical applications. Handbook of Experimental Pharmacology, 256, 237–264. - PubMed
Publication types
MeSH terms
- VSports注册入口 - Actions
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- VSports在线直播 - Actions
- "VSports app下载" Actions
- "V体育2025版" Actions
- VSports app下载 - Actions
- V体育官网 - Actions
- Actions (V体育安卓版)
- Actions (V体育平台登录)
"V体育官网入口" Substances
- "VSports在线直播" Actions
- Actions (V体育平台登录)
- VSports最新版本 - Actions
Grants and funding
"V体育2025版" LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports注册入口" Research Materials

