VSports - Bile acid-based therapies for non-alcoholic steatohepatitis and alcoholic liver disease
- PMID: 32355674
- PMCID: "VSports最新版本" PMC7188552
- DOI: 10.21037/hbsn.2019.09.03
Bile acid-based therapies for non-alcoholic steatohepatitis and alcoholic liver disease
Abstract
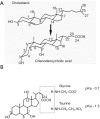
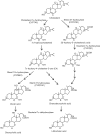
Bile acids are synthesized from cholesterol only in hepatocytes. Bile acids circulating in the enterohepatic system act as physiological detergent molecules to help solubilize biliary cholesterol and emulsify dietary lipids and fat-soluble vitamins in small intestine VSports手机版. Bile acids are signaling molecules that activate nuclear receptor farnesoid X receptor (FXR) and cell surface G protein-coupled receptor TGR5. FXR critically regulates bile acid homeostasis by mediating bile acid feedback inhibition of hepatic bile acid synthesis. In addition, bile acid-activated cellular signaling pathways regulate metabolic homeostasis, immunity, and cell proliferation in various metabolically active organs. In the small and large intestine, gut bacterial enzymes modify primary bile acids to generate secondary bile acids to help shape the bile acid pool composition and subsequent biological effects. In turn, bile acids exhibit anti-microbial properties and modulate gut microbiota to influence host metabolism and immunity. Currently, bile acid-based therapies including systemic and intestine-restricted FXR agonists, TGR5 agonists, fibroblast growth factor 19 analogue, intestine FXR antagonists, and intestine apical sodium-bile acid transporter (ASBT) inhibitors have been developed as promising treatments for non-alcoholic steatohepatitis (NASH). These pharmacological agents improved metabolic and inflammatory disorders via distinct mechanisms of action that are subjects of extensive research interest. More recently, human and experimental alcoholic liver disease (ALD) has been associated with disrupted bile acid homeostasis. In additional, new findings showed that targeting bile acid metabolism and signaling may be promising therapeutic approaches for treating ALD. .
Keywords: Bile acid; alcoholic liver disease (ALD); farnesoid X receptor (FXR); microbiota; non-alcoholic steatohepatitis (NASH). V体育安卓版.
2020 Hepatobiliary Surgery and Nutrition V体育ios版. All rights reserved. .
Conflict of interest statement
Conflicts of Interest: The series “Gut Microbiome and Liver Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare VSports最新版本.
Figures
References
-
- Falany CN, Johnson MR, Barnes S, et al. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J Biol Chem 1994;269:19375-9. - PubMed
-
- Staudinger JL, Goodwin B, Jones SA, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A 2001;98:3369-74. 10.1073/pnas.051551698 - DOI (VSports手机版) - PMC - PubMed
Publication types
- Actions (VSports注册入口)
Grants and funding
LinkOut - more resources
Full Text Sources
