Evaluation of Cyclophosphamide/GVAX Pancreas Followed by Listeria-Mesothelin (CRS-207) with or without Nivolumab in Patients with Pancreatic Cancer (V体育官网)
- PMID: 32273276
- PMCID: PMC7727397
- DOI: V体育官网 - 10.1158/1078-0432.CCR-19-3978
VSports app下载 - Evaluation of Cyclophosphamide/GVAX Pancreas Followed by Listeria-Mesothelin (CRS-207) with or without Nivolumab in Patients with Pancreatic Cancer
Abstract
Purpose: Two studies in previously treated metastatic pancreatic cancer have been completed combining GVAX pancreas vaccine (GM-CSF-secreting allogeneic pancreatic tumor cells) with cyclophosphamide (Cy) and CRS-207 (live, attenuated Listeria monocytogenes-expressing mesothelin). In the current study, we compared Cy/GVAX followed by CRS-207 with (Arm A) or without nivolumab (Arm B). VSports手机版.
Patients and methods: Patients with pancreatic adenocarcinoma who received one prior therapy for metastatic disease and RECIST measurable disease were randomized 1:1 to receive treatment on Arm A or Arm B V体育安卓版. The primary objective was to compare overall survival (OS) between the arms. Additional objectives included assessment of progression-free survival, safety, tumor responses, CA19-9 responses, and immunologic correlates. .
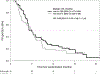
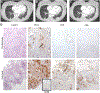
Results: Ninety-three patients were treated (Arm A, 51; Arm B, 42). The median OS in Arms A and B were 5. 9 [95% confidence interval (CI), 4. 7-8. 6] and 6. 1 (95% CI, 3. 5-7. 0) months, respectively, with an HR of 0 V体育ios版. 86 (95% CI, 0. 55-1. 34). Objective responses were seen in 3 patients using immune-related response criteria (4%, 2/51, Arm A; 2%, 1/42, Arm B). The grade ≥3 related adverse event rate, whereas higher in Arm A (35. 3% vs. 11. 9%) was manageable. Changes in the microenvironment, including increase in CD8+ T cells and a decrease in CD68+ myeloid cells, were observed in long-term survivors in Arm A only. .
Conclusions: Although the study did not meet its primary endpoint of improvement in OS of Arm A over Arm B, the OS was comparable with standard therapy VSports最新版本. Objective responses and immunologic changes in the tumor microenvironment were evident. .
©2020 American Association for Cancer Research. V体育平台登录.
Conflict of interest statement
Conflict of Interest Statement: T. Tsujukawa is a consultant for Konica Minolta and Ono Pharmaceutical. T. Crocenzi has received research support from Bristol-Myers Squibb and AstraZeneca. R. A. Anders is on the scientific advisory board or receives research support from Merck, Bristol-Myers Squibb, FLX Bio, Incyte and Adaptive Biotech. E. J. Fertig is a consultant for Champions Oncology. K. A. Reiss has research support from Clovis, Bristol-Myer Squibb, Tesaro and Lilly Oncology. R. H. Vonderheide reports having received consulting fees or honoraria from Apexigen, AstraZeneca, Celgene, Genentech, Janssen, Lilly, Medimmune, Merck and Verastem. He has received research funding from Apexigen, Fibrogen, Inovio, Janssen, and Lilly. He is an inventor on a licensed patent relating to cancer cellular immunotherapy and receives royalties from Children’s Hospital Boston for a licensed research-only monoclonal antibody VSports注册入口. A. H. Ko has received research support from Aduro Biotech and Bristol-Myers Squibb. G. A. Fisher serves on advisory boards for or received honoraria from Merck, Roche/Genentech, Aduro Biotech, and FortySeven. He serves on Data Safety Monitoring Committees for Celgene, CytomX, Silenseed, Terumo, and AstraZeneca. D. G. Brockstedt is a former employee of and is a shareholder in Aduro Biotech. L. M. Coussens is a paid consultant for Cell Signaling Technologies, received reagent and/or research support from Plexxikon Inc. , Pharmacyclics, Inc. , Acerta Pharma, LLC, Deciphera Pharmaceuticals, LLC, Genentech, Inc. , Roche Glycart AG, Syndax Pharmaceuticals Inc. , and NanoString Technologies, and is a member of the Scientific Advisory Boards of Syndax Pharmaceuticals, Carisma Therapeutics, Zymeworks, Inc, Verseau Therapeutics, and Cytomix Therapeutics, Inc. E. M. Jaffee and Johns Hopkins University have the potential receive royalties from Aduro Biotech which owns the license to GVAX and Listeria Monocytogenes-mesothelin. E. Jaffee is the Chief Medical Advisor for the Lustgarten Foundation and is active on the scientific advisory boards of Genocea, Adaptive Biotech, DragonFly, CSTONE, Achilles, Parker Institute and the National Cancer Advisory Board. She receives research funding from Aduro Biotech, Amgen, Bristol-Myers Squibb, Hertix and Corvus. D. Le serves on the advisory boards for Merck and Bristol-Myers Squibb and has received research funding from Merck, Bristol-Myers Squibb, Aduro Biotech, Curegenix, Medivir and Nouscom. She has received speaking honoraria from Merck.
"VSports手机版" Figures

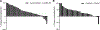

References
-
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2(7):616–31. - V体育安卓版 - PMC - PubMed
-
- Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38(1):1–11. - VSports app下载 - PMC - PubMed
-
- Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18(3):858–68. - PMC - PubMed
-
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33(12):1325–33. - PMC (V体育2025版) - PubMed
Publication types
- V体育官网 - Actions
- "V体育ios版" Actions
- VSports在线直播 - Actions
- "V体育官网" Actions
- "VSports注册入口" Actions
- VSports注册入口 - Actions
MeSH terms
- "V体育ios版" Actions
- Actions (VSports在线直播)
- VSports注册入口 - Actions
- Actions (V体育ios版)
- Actions (VSports app下载)
- V体育安卓版 - Actions
- "V体育2025版" Actions
- V体育官网 - Actions
- V体育2025版 - Actions
- "V体育官网" Actions
- Actions (V体育官网入口)
- "V体育ios版" Actions
V体育安卓版 - Substances
- V体育平台登录 - Actions
- "V体育ios版" Actions
- Actions (V体育安卓版)
- VSports app下载 - Actions
Grants and funding
"V体育官网" LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育ios版" Medical
Research Materials (VSports最新版本)

