V体育官网 - RIG-I-based immunotherapy enhances survival in preclinical AML models and sensitizes AML cells to checkpoint blockade
- PMID: 31740809
- PMCID: "VSports app下载" PMC7214254
- DOI: 10.1038/s41375-019-0639-x
RIG-I-based immunotherapy enhances survival in preclinical AML models and sensitizes AML cells to checkpoint blockade
Abstract
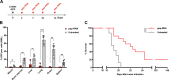
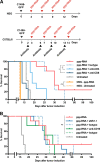
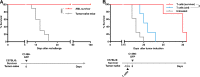
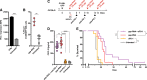
Retinoic acid-inducible gene-I (RIG-I) is a cytoplasmic immune receptor sensing viral RNA. It triggers the release of type I interferons (IFN) and proinflammatory cytokines inducing an adaptive cellular immune response. We investigated the therapeutic potential of systemic RIG-I activation by short 5'-triphosphate-modified RNA (ppp-RNA) for the treatment of acute myeloid leukemia (AML) in the syngeneic murine C1498 AML tumor model. ppp-RNA treatment significantly reduced tumor burden, delayed disease onset and led to complete remission including immunological memory formation in a substantial proportion of animals. Therapy-induced tumor rejection was dependent on CD4+ and CD8+ T cells, but not on NK or B cells, and relied on intact IFN and mitochondrial antiviral signaling protein (MAVS) signaling in the host. Interestingly, ppp-RNA treatment induced programmed death ligand 1 (PD-L1) expression on AML cells and established therapeutic sensitivity to anti-PD-1 checkpoint blockade in vivo. In immune-reconstituted humanized mice, ppp-RNA treatment reduced the number of patient-derived xenografted (PDX) AML cells in blood and bone marrow while concomitantly enhancing CD3+ T cell counts in the respective tissues. Due to its ability to establish a state of full remission and immunological memory, our findings show that ppp-RNA treatment is a promising strategy for the immunotherapy of AML. VSports手机版.
Conflict of interest statement
The authors declare no competing financial interests. Parts of this work have been performed for the doctoral theses of MR, EH, HM, and MZ at the LMU Munich V体育安卓版.
Figures

References (VSports)
-
- Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, et al. 5′-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med. 2008;14:1256–63. doi: 10.1038/nm.1887. - V体育2025版 - DOI - PubMed
-
- Tormo D, Checińska A, Alonso-Curbelo D, Pérez-Guijarro E, Cañón E, Riveiro-Falkenbach E, et al. Targeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cells. Cancer Cell. 2009;16:103–14. doi: 10.1016/j.ccr.2009.07.004. - V体育安卓版 - DOI - PMC - PubMed
Publication types
MeSH terms
- Actions (VSports app下载)
- Actions (V体育官网入口)
- "VSports最新版本" Actions
- Actions (VSports注册入口)
- V体育ios版 - Actions
- "VSports注册入口" Actions
- Actions (VSports在线直播)
- "VSports最新版本" Actions
- Actions (V体育安卓版)
- VSports最新版本 - Actions
- "VSports最新版本" Actions
- "VSports" Actions
- Actions (V体育2025版)
Substances
- V体育ios版 - Actions
VSports手机版 - LinkOut - more resources
Full Text Sources
"VSports" Medical
Molecular Biology Databases
"VSports手机版" Research Materials
Miscellaneous

