Caspase-8, receptor-interacting protein kinase 1 (RIPK1), and RIPK3 regulate retinoic acid-induced cell differentiation and necroptosis
- PMID: 31659279
- PMCID: VSports在线直播 - PMC7206185
- DOI: "VSports" 10.1038/s41418-019-0434-2
Caspase-8, receptor-interacting protein kinase 1 (RIPK1), and RIPK3 regulate retinoic acid-induced cell differentiation and necroptosis
Abstract
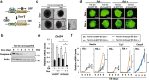
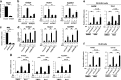
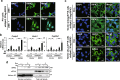
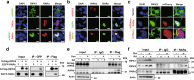
Among caspase family members, Caspase-8 is unique, with associated critical activities to induce and suppress death receptor-mediated apoptosis and necroptosis, respectively. Caspase-8 inhibits necroptosis by suppressing the function of receptor-interacting protein kinase 1 (RIPK1 or RIP1) and RIPK3 to activate mixed lineage kinase domain-like (MLKL) VSports手机版. Disruption of Caspase-8 expression causes embryonic lethality in mice, which is rescued by depletion of either Ripk3 or Mlkl, indicating that the embryonic lethality is caused by activation of necroptosis. Here, we show that knockdown of Caspase-8 expression in embryoid bodies derived from ES cells markedly enhances retinoic acid (RA)-induced cell differentiation and necroptosis, both of which are dependent on Ripk1 and Ripk3; however, the enhancement of RA-induced cell differentiation is independent of Mlkl and necrosome formation. RA treatment obviously enhanced the expression of RA-specific target genes having the retinoic acid response element (RARE) in their promoter regions to induce cell differentiation, and induced marked expression of RIPK1, RIPK3, and MLKL to stimulate necroptosis. Caspase-8 knockdown induced RIPK1 and RIPK3 to translocate into the nucleus and to form a complex with RA receptor (RAR), and RAR interacting with RIPK1 and RIPK3 showed much stronger binding activity to RARE than RAR without RIPK1 or RIPK3. In Caspase-8-deficient as well as Caspase-8- and Mlkl-deficient mouse embryos, the expression of RA-specific target genes was obviously enhanced. Thus, Caspase-8, RIPK1, and RIPK3 regulate RA-induced cell differentiation and necroptosis both in vitro and in vivo. .
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Alnemri E. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64:33–42. - VSports - PubMed
-
- Chinnaiyan AM, O’Rourke K, Lane BR, Dixit VM. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275:1122–6. - PubMed
-
- Green D. Apoptotic pathways: ten minutes to dead. Cell. 2005;121:671–4. - PubMed
-
- Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–6. - PubMed
Publication types (V体育官网)
MeSH terms
- VSports最新版本 - Actions
- V体育ios版 - Actions
- VSports app下载 - Actions
- "VSports手机版" Actions
- V体育官网 - Actions
- "V体育2025版" Actions
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- "VSports最新版本" Actions
- "V体育官网" Actions
- Actions (V体育官网入口)
- Actions (VSports app下载)
Substances
- "V体育2025版" Actions
- V体育ios版 - Actions
- V体育ios版 - Actions
- VSports最新版本 - Actions
LinkOut - more resources (V体育官网入口)
"VSports在线直播" Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

